Responsable : Fabrice FLEURY
Projets et objectifs:
L’objectif de notre équipe est de comprendre les mécanismes moléculaires et biochimiques de la réparation de l’ADN, notamment par recombinaison homologue (RH), et d’explorer certaines voies de réparation impliquées dans les processus de résistance aux traitements anticancéreux.
Nous étudions en particulier Rad51, protéine clef de la RH fréquemment surexprimée dans les cellules cancéreuses et qui est à l’origine de résistance aux radio- et chimio-thérapies anticancéreuses.
Les interactions impliquant des protéines de réparation de l’ADN sont étudiées par criblage à haut débit (Puces à protéines). Leur caractérisation et leur analyse structure-fonction sont abordées par un ensemble d’outils biophysiques (disponibles via la plate-forme IMPACT) parfaitement maîtrisés par notre équipe. Les informations moléculaires ainsi extraites sont ensuite exploitées pour mieux comprendre les régulations au niveau cellulaire. Cette interface moléculaire-cellulaire est l’un des points forts qui caractérise notre équipe.
Mots-clés : biochimie, modifications post-traductionnelles, RAD51, réparation ADN
Membres
Anciens membres de l'équipe
- Mohamad ALAOUID, Doctorant
- Brendan ALLIGAND, Doctorant
- Iman AMRANI , Doctorant
- Gwenola BOUCHER, Maître de conférences
- Thomas CHABOT, Doctorant
- Gwennina CUEFF, Technicienne de la recherche
- Alexandre DEMEYER , Doctorant
- Titouan JAUNET-LAHARY, Doctorant
- Camille JUBELIN, Doctorant
- Daria KLUSA, Post-doctorante
- Florian LAFONT, Doctorant
- Joshua MOUNTFORD, Doctorant
- Denis VELIC, Doctorant
Projets
Publications
2 publications
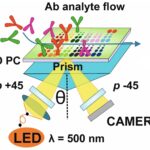
Nifontova, Galina; Charlier, Cathy; Ayadi, Nizar; Fleury, Fabrice; Karaulov, Alexander; Sukhanova, Alyona; Nabiev, Igor
Photonic Crystal Surface Mode Real-Time Imaging of RAD51 DNA Repair Protein Interaction with the ssDNA Substrate Article de journal
Dans: Biosensors, vol. 14, no. 1, 2024, ISSN: 2079-6374.
@article{bios14010043,
title = {Photonic Crystal Surface Mode Real-Time Imaging of RAD51 DNA Repair Protein Interaction with the ssDNA Substrate},
author = {Galina Nifontova and Cathy Charlier and Nizar Ayadi and Fabrice Fleury and Alexander Karaulov and Alyona Sukhanova and Igor Nabiev},
url = {https://www.mdpi.com/2079-6374/14/1/43},
doi = {10.3390/bios14010043},
issn = {2079-6374},
year = {2024},
date = {2024-01-01},
urldate = {2024-01-01},
journal = {Biosensors},
volume = {14},
number = {1},
abstract = {Photonic crystals (PCs) are promising tools for label-free sensing in drug discovery screening, diagnostics, and analysis of ligand-receptor interactions. Imaging of PC surface modes has emerged as a novel approach to the detection of multiple binding events at the sensor surface. PC surface modification and decoration with recognition units yield an interface providing the highly sensitive detection of cancer biomarkers, antibodies, and oligonucleotides. The RAD51 protein plays a central role in DNA repair via the homologous recombination pathway. This recombinase is essential for the genome stability and its overexpression is often correlated with aggressive cancer. RAD51 is therefore a potential target in the therapeutic strategy for cancer. Here, we report the designing of a PC-based array sensor for real-time monitoring of oligonucleotide-RAD51 recruitment by means of surface mode imaging and validation of the concept of this approach. Our data demonstrate that the designed biosensor ensures the highly sensitive multiplexed analysis of association-dissociation events and detection of the biomarker of DNA damage using a microfluidic PC array. The obtained results highlight the potential of the developed technique for testing the functionality of candidate drugs, discovering new molecular targets and drug entities. This paves the way to further adaption and bioanalytical use of the biosensor for high-content screening to identify new DNA repair inhibitor drugs targeting the RAD51 nucleoprotein filament or to discover new molecular targets.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Jubelin, Camille; Muñoz-Garcia, Javier; Ollivier, Emilie; Cochonneau, Denis; Vallette, François; Heymann, Marie-Françoise; Oliver, Lisa; Heymann, Dominique
Identification of MCM4 and PRKDC as new regulators of osteosarcoma cell dormancy based on 3D cell cultures Article de journal
Dans: Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, p. 119660, 2024, ISSN: 0167-4889.
@article{JUBELIN2024119660,
title = {Identification of MCM4 and PRKDC as new regulators of osteosarcoma cell dormancy based on 3D cell cultures},
author = {Camille Jubelin and Javier Muñoz-Garcia and Emilie Ollivier and Denis Cochonneau and François Vallette and Marie-Françoise Heymann and Lisa Oliver and Dominique Heymann},
url = {https://www.sciencedirect.com/science/article/pii/S016748892400003X},
doi = {https://doi.org/10.1016/j.bbamcr.2024.119660},
issn = {0167-4889},
year = {2024},
date = {2024-01-01},
urldate = {2024-01-01},
journal = {Biochimica et Biophysica Acta (BBA) - Molecular Cell Research},
pages = {119660},
abstract = {Dormancy is a potential way for tumors to develop drug resistance and escape treatment. However, the mechanisms involved in cancer dormancy remain poorly understood. This is mainly because there is no in vitro culture model making it possible to spontaneously induce dormancy. In this context, the present work proposes the use of three-dimensional (3D) spheroids developed from osteosarcoma cell lines as a relevant model for studying cancer dormancy. MNNG-HOS, SaOS-2, 143B, MG-63, U2OS and SJSA-1 cell lines were cultured in 3D using the Liquid Overlay Technique (LOT). Dormancy was studied by staining cancer cells with a lipophilic dye (DiD), and long-term DiD+ cells were considered as dormant cancer cells. The role of the extracellular matrix in inducing dormancy was investigated by embedding cells into methylcellulose or Geltrex™. Gene expression of DiD+ cells was assessed with a Nanostring™ approach and the role of the genes detected in dormancy was validated by a transient down-expression model using siRNA treatment. Proliferation was measured using fluorescence microscopy and the xCELLigence technology. We observed that MNNG-HOS, 143B and MG-G3 cell lines had a reduced proliferation rate in 3D compared to 2D. U2OS cells had an increased proliferation rate when they were cultured in Geltrex™ compared to other 3D culture methods. Using 3D cultures, a transcriptomic signature of dormancy was obtained and showed a decreased expression of 18 genes including ETV4, HELLS, ITGA6, MCM4, PRKDC, RAD21 and UBE2T. The treatment with siRNA targeting these genes showed that cancer cell proliferation was reduced when the expression of ETV4 and MCM4 were decreased, whereas proliferation was increased when the expression of RAD21 was decreased. 3D culture facilitates the maintenance of dormant cancer cells characterized by a reduced proliferation and less differential gene expression as compared to proliferative cells. Further studies of the genes involved has enabled us to envisage their role in regulating cell proliferation.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
13 publications
Panez-Toro, Isidora; Heymann, Dominique; Gouin, François; Amiaud, Jérôme; Heymann, Marie-Françoise; Córdova, Luis A.
Roles of inflammatory cell infiltrate in periprosthetic osteolysis Article de journal
Dans: Frontiers in Immunology, vol. 14, p. 1310262, 2023, ISSN: 1664-3224.
@article{panez-toro_roles_2023,
title = {Roles of inflammatory cell infiltrate in periprosthetic osteolysis},
author = {Isidora Panez-Toro and Dominique Heymann and François Gouin and Jérôme Amiaud and Marie-Françoise Heymann and Luis A. Córdova},
url = {https://www.frontiersin.org/articles/10.3389/fimmu.2023.1310262/full},
doi = {10.3389/fimmu.2023.1310262},
issn = {1664-3224},
year = {2023},
date = {2023-12-01},
urldate = {2023-12-01},
journal = {Frontiers in Immunology},
volume = {14},
pages = {1310262},
abstract = {Classically, particle-induced periprosthetic osteolysis at the implant–bone interface has explained the aseptic loosening of joint replacement. This response is preceded by triggering both the innate and acquired immune response with subsequent activation of osteoclasts, the bone-resorbing cells. Although particle-induced periprosthetic osteolysis has been considered a foreign body chronic inflammation mediated by myelomonocytic-derived cells, current reports describe wide heterogeneous inflammatory cells infiltrating the periprosthetic tissues. This review aims to discuss the role of those non-myelomonocytic cells in periprosthetic tissues exposed to wear particles by showing original data. Specifically, we discuss the role of T cells (CD3+
, CD4+, and CD8+) and B cells (CD20+) coexisting with CD68+/TRAP−multinucleated giant cells associated with both polyethylene and metallic particles infiltrating retrieved periprosthetic membranes. This review contributes valuable insight to support the complex cell and molecular mechanisms behind the aseptic loosening theories of orthopedic implants.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
, CD4+, and CD8+) and B cells (CD20+) coexisting with CD68+/TRAP−multinucleated giant cells associated with both polyethylene and metallic particles infiltrating retrieved periprosthetic membranes. This review contributes valuable insight to support the complex cell and molecular mechanisms behind the aseptic loosening theories of orthopedic implants.
Dubois, Nolwenn; Muñoz-Garcia, Javier; Heymann, Dominique; Renodon-Cornière, Axelle
High glucose exposure drives intestinal barrier dysfunction by altering its morphological, structural and functional properties. Article de journal
Dans: Biochemical pharmacology, vol. 216, p. 115765, 2023, ISSN: 1873-2968 0006-2952, (Place: England).
@article{dubois_high_2023,
title = {High glucose exposure drives intestinal barrier dysfunction by altering its morphological, structural and functional properties.},
author = {Nolwenn Dubois and Javier Muñoz-Garcia and Dominique Heymann and Axelle Renodon-Cornière},
doi = {10.1016/j.bcp.2023.115765},
issn = {1873-2968 0006-2952},
year = {2023},
date = {2023-10-01},
urldate = {2023-10-01},
journal = {Biochemical pharmacology},
volume = {216},
pages = {115765},
abstract = {High dietary glucose consumption and hyperglycemia can result in chronic complications. Several studies suggest that high glucose (HG) induces dysfunction of the intestinal barrier. However, the precise changes remain unclear. In our study, we used in vitro models composed of Caco-2 and/or HT29-MTX cells in both monoculture and co-culture to assess the effects of long-term HG exposure on the morphological, structural, and functional properties of the intestinal barrier. Cells were grown in medium containing normal physiologic glucose (NG, 5.5 mM) or a clinically relevant HG (25 mM) concentration until 21 days. Results demonstrated that HG induced morphological changes, with the layers appearing denser and less organized than under physiological conditions, which is in accordance with the increased migration capacity of Caco-2 cells and proliferation properties of HT29-MTX cells. Although we mostly observed a small decrease in mRNA and protein expressions of three junction proteins (ZO-1, OCLN and E-cad) in both Caco-2 and HT29-MTX cells cultured in HG medium, confocal microscopy showed that HG induced a remarkable reduction in their immunofluorescence intensity, triggering disruption of their associated structural network. In addition, we highlighted that HG affected different functionalities (permeability, mucus production and alkaline phosphatase activity) of monolayers with Caco-2 and HT29-MTX cells. Interestingly, these alterations were stronger in co-culture than in monoculture, suggesting a cross-relationship between enterocytes and goblet cells. Controlling hyperglycemia remains a major therapeutical method for reducing damage to the intestinal barrier and improving therapies.},
note = {Place: England},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Pu, Yi; Li, Lu; Peng, Haoning; Liu, Lunxu; Heymann, Dominique; Robert, Caroline; Vallette, François; Shen, Shensi
Drug-tolerant persister cells in cancer: the cutting edges and future directions Article de journal
Dans: Nature Reviews Clinical Oncology, 2023, ISSN: 1759-4782.
@article{pu_drug-tolerant_2023,
title = {Drug-tolerant persister cells in cancer: the cutting edges and future directions},
author = {Yi Pu and Lu Li and Haoning Peng and Lunxu Liu and Dominique Heymann and Caroline Robert and François Vallette and Shensi Shen},
url = {https://doi.org/10.1038/s41571-023-00815-5},
doi = {10.1038/s41571-023-00815-5},
issn = {1759-4782},
year = {2023},
date = {2023-09-01},
urldate = {2023-09-01},
journal = {Nature Reviews Clinical Oncology},
abstract = {Drug-tolerant persister (DTP) cell populations were originally discovered in antibiotic-resistant bacterial biofilms. Similar populations with comparable features have since been identified among cancer cells and have been linked with treatment resistance that lacks an underlying genomic alteration. Research over the past decade has improved our understanding of the biological roles of DTP cells in cancer, although clinical knowledge of the role of these cells in treatment resistance remains limited. Nonetheless, targeting this population is anticipated to provide new treatment opportunities. In this Perspective, we aim to provide a clear definition of the DTP phenotype, discuss the underlying characteristics of these cells, their biomarkers and vulnerabilities, and encourage further research on DTP cells that might improve our understanding and enable the development of more effective anticancer therapies.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Antoine, Babuty; Boisseau, Pierre; Drillaud, Nicolas; Eveillard, Marion; Fouassier, Marc
MYH9-related disease: Assessment of the pathogenicity of a new mutation Article de journal
Dans: EJHaem, vol. 4, no. 3, p. 869–871, 2023, ISSN: 2688-6146.
@article{pmid37601883,
title = {MYH9-related disease: Assessment of the pathogenicity of a new mutation},
author = {Babuty Antoine and Pierre Boisseau and Nicolas Drillaud and Marion Eveillard and Marc Fouassier},
doi = {10.1002/jha2.715},
issn = {2688-6146},
year = {2023},
date = {2023-08-01},
urldate = {2023-08-01},
journal = {EJHaem},
volume = {4},
number = {3},
pages = {869--871},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Panez-Toro, Isidora; Muñoz-García, Javier; Vargas-Franco, Jorge W.; Renodon-Cornière, Axelle; Heymann, Marie-Françoise; Lézot, Frédéric; Heymann, Dominique
Advances in Osteosarcoma Article de journal
Dans: Current Osteoporosis Reports, 2023, ISSN: 1544-1873, 1544-2241.
@article{panez-toro_advances_2023,
title = {Advances in Osteosarcoma},
author = {Isidora Panez-Toro and Javier Muñoz-García and Jorge W. Vargas-Franco and Axelle Renodon-Cornière and Marie-Françoise Heymann and Frédéric Lézot and Dominique Heymann},
url = {https://link.springer.com/10.1007/s11914-023-00803-9},
doi = {10.1007/s11914-023-00803-9},
issn = {1544-1873, 1544-2241},
year = {2023},
date = {2023-06-01},
urldate = {2023-06-01},
journal = {Current Osteoporosis Reports},
abstract = {Purpose of Review
This article gives a brief overview of the most recent developments in osteosarcoma treatment, including targeting of signaling pathways, immune checkpoint inhibitors, drug delivery strategies as single or combined approaches, and the identification of new therapeutic targets to face this highly heterogeneous disease.
Recent Findings
Osteosarcoma is one of the most common primary malignant bone tumors in children and young adults, with a high risk of bone and lung metastases and a 5-year survival rate around 70% in the absence of metastases and 30% if metastases are detected at the time of diagnosis. Despite the novel advances in neoadjuvant chemotherapy, the effective treatment for osteosarcoma has not improved in the last 4 decades. The emergence of immunotherapy has transformed the paradigm of treatment, focusing therapeutic strategies on the potential of immune checkpoint inhibitors. However, the most recent clinical trials show a slight improvement over the conventional polychemotherapy scheme.
Summary
The tumor microenvironment plays a crucial role in the pathogenesis of osteosarcoma by controlling the tumor growth, the metastatic process and the drug resistance and paved the way of new therapeutic options that must be validated by accurate pre-clinical studies and clinical trials.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
This article gives a brief overview of the most recent developments in osteosarcoma treatment, including targeting of signaling pathways, immune checkpoint inhibitors, drug delivery strategies as single or combined approaches, and the identification of new therapeutic targets to face this highly heterogeneous disease.
Recent Findings
Osteosarcoma is one of the most common primary malignant bone tumors in children and young adults, with a high risk of bone and lung metastases and a 5-year survival rate around 70% in the absence of metastases and 30% if metastases are detected at the time of diagnosis. Despite the novel advances in neoadjuvant chemotherapy, the effective treatment for osteosarcoma has not improved in the last 4 decades. The emergence of immunotherapy has transformed the paradigm of treatment, focusing therapeutic strategies on the potential of immune checkpoint inhibitors. However, the most recent clinical trials show a slight improvement over the conventional polychemotherapy scheme.
Summary
The tumor microenvironment plays a crucial role in the pathogenesis of osteosarcoma by controlling the tumor growth, the metastatic process and the drug resistance and paved the way of new therapeutic options that must be validated by accurate pre-clinical studies and clinical trials.
Loussouarn, Delphine; Oliver, Lisa; Salaud, Celine; Samarut, Edouard; Bourgade, Raphaël; Béroud, Christophe; Morenton, Emilie; Heymann, Dominique; Vallette, Francois M.
Spatial Distribution of Immune Cells in Primary and Recurrent Glioblastoma: A Small Case Study Article de journal
Dans: Cancers, vol. 15, no. 12, p. 3256, 2023, ISSN: 2072-6694.
@article{loussouarn_spatial_2023,
title = {Spatial Distribution of Immune Cells in Primary and Recurrent Glioblastoma: A Small Case Study},
author = {Delphine Loussouarn and Lisa Oliver and Celine Salaud and Edouard Samarut and Raphaël Bourgade and Christophe Béroud and Emilie Morenton and Dominique Heymann and Francois M. Vallette},
url = {https://www.mdpi.com/2072-6694/15/12/3256},
doi = {10.3390/cancers15123256},
issn = {2072-6694},
year = {2023},
date = {2023-06-01},
urldate = {2023-06-01},
journal = {Cancers},
volume = {15},
number = {12},
pages = {3256},
abstract = {Only a minority of patients with glioblastoma (GBM) respond to immunotherapy, and always only partially. There is a lack of knowledge on immune distribution in GBM and in its tumor microenvironment (TME). To address the question, we used paired primary and recurrent tumors from GBM patients to study the composition and the evolution of the immune landscape upon treatment. We studied the expression of a handful of immune markers (CD3, CD8, CD68, PD-L1 and PD-1) in GBM tissues in 15 paired primary and recurrent GBM. In five selected patients, we used Nanostring Digital Spatial Profiling (DSP) to obtain simultaneous assessments of multiple biomarkers both within the tumor and the microenvironment in paired primary and recurrent GBM. Our results suggest that the evolution of the immune landscape between paired primary and recurrent GBM tumors is highly heterogeneous. However, our study identifies B3-H7 and HLA-DR as potential targets in primary and recurrent GBM. Spatial profiling of immune markers from matched primary and recurrent GBM shows a nonlinear complex evolution during the progression of cancer. Nonetheless, our study demonstrated a global increase in macrophages, and revealed differential localization of some markers, such as B7-H3 and HLA-DR, between GBM and its TME.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Metz, Raphaël; Rauscher, Aurore; Vaugier, Loïg; Supiot, Stéphane; Drouet, Franck; Campion, Loic; Rousseau, Caroline
Comparison of Hormone-Sensitive Oligorecurrent Prostate Cancer Patients Based on Routine Use of Choline and/or PSMA PET/CT to Guide Metastasis-Directed Therapy Article de journal
Dans: Cancers, vol. 15, no. 6, 2023, ISSN: 2072-6694.
@article{cancers15061898,
title = {Comparison of Hormone-Sensitive Oligorecurrent Prostate Cancer Patients Based on Routine Use of Choline and/or PSMA PET/CT to Guide Metastasis-Directed Therapy},
author = {Raphaël Metz and Aurore Rauscher and Loïg Vaugier and Stéphane Supiot and Franck Drouet and Loic Campion and Caroline Rousseau},
url = {https://www.mdpi.com/2072-6694/15/6/1898},
doi = {10.3390/cancers15061898},
issn = {2072-6694},
year = {2023},
date = {2023-03-22},
urldate = {2023-01-01},
journal = {Cancers},
volume = {15},
number = {6},
abstract = {Background: In hormone-sensitive oligorecurrent prostate cancer (PC), the literature showed [68Ga]Ga-PSMA (PSMA) and [18F]F-choline (FCH) PET/CT can successfully guide metastasis-directed therapies (MDT). This observational retrospective study aimed to explore, in routine use, the impact of FCH or PSMA PET/CT in guiding MDT for hormone-sensitive oligometastatic PC at different recurrences. Methods: In 2017-2020, patients initially treated with radical prostatectomy but, in biochemical recurrence (with PSA ≤ 2 ng/mL), diagnosed as oligometastatic based on FCH or PSMA PET/CT, were identified. MDT was stereotactic body radiotherapy (SBRT), elective nodal or prostate bed radiotherapy ± boost and ± androgen deprivation therapy (ADT). The primary endpoint was biochemical relapse-free survival (BR-FS), defined as a PSA increase ≥ 0.2 ng/mL above the nadir and increasing over two successive samples and the secondaries were ADT-free survival (ADT-FS). Results: 123 patients (70 PSMA and 53 FCH) were included. The median follow-up was 42.2 months. The median BR-FS was 24.7 months in the PSMA group versus 13.0 months in the FCH group (p = 0.008). Similarly, ADT-FS (p = 0.001) was longer in patients in the PSMA group. In multivariate analysis, a short PSA doubling time before imaging (p = 0.005) and MDT with SBRT (p = 0.001) were poor prognostic factors for BR-FS. Conclusions: Routine use of FCH or PSMA PET/CT in hormone-sensitive PC showed an advantage for using PSMA PET/CT to guide MDT in terms of BR-FS and ADT-FS in patients with low PSA value. Prospective studies are needed to confirm these hypotheses.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Demeyer, Alexandre; Fonteneau, Lucie; Liennard, Marion; Foyer, Claire; Weigel, Pierre; Laurent, Adèle; Lebreton, Jacques; Fleury, Fabrice; Mathé-Allainmat, Monique
Synthesis and Biological Evaluation of DIDS Analogues as Efficient Inhibitors of RAD51 Involved in Homologous Recombination Article de journal
Dans: Bioorg Med Chem Lett, p. 129261, 2023, ISSN: 1464-3405.
@article{pmid36990245,
title = {Synthesis and Biological Evaluation of DIDS Analogues as Efficient Inhibitors of RAD51 Involved in Homologous Recombination},
author = {Alexandre Demeyer and Lucie Fonteneau and Marion Liennard and Claire Foyer and Pierre Weigel and Adèle Laurent and Jacques Lebreton and Fabrice Fleury and Monique Mathé-Allainmat},
doi = {10.1016/j.bmcl.2023.129261},
issn = {1464-3405},
year = {2023},
date = {2023-03-01},
urldate = {2023-03-01},
journal = {Bioorg Med Chem Lett},
pages = {129261},
abstract = {RAD51 is a pivotal protein of the homologous recombination DNA repair pathway, and is overexpressed in some cancer cells, disrupting then the efficiency of cancer-treatments. The development of RAD51 inhibitors appears as a promising solution to restore these cancer cells sensitization to radio- or chemotherapy. From a small molecule identified as a modulator of RAD51, the 4,4'-diisothiocyanostilbene-2,2'-disulfonic acid (DIDS), two series of analogues with small or bulky substituents on the aromatic parts of the stilbene moiety were prepared for a structure-activity relationship study. Three compounds, the cyano analogue (12), and benzamide (23) or phenylcarbamate (29) analogues of DIDS were characterized as novel potent RAD51 inhibitors with HR inhibition in the micromolar range.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Saade, Gaëlle; Bogaerts, Eva; Chiavassa, Sophie; Blain, Guillaume; Delpon, Grégory; Evin, Manon; Ghannam, Youssef; Haddad, Ferid; Haustermans, Karin; Koumeir, Charbel; others,
Ultrahigh-Dose-Rate Proton Irradiation Elicits Reduced Toxicity in Zebrafish Embryos Article de journal
Dans: Advances in Radiation Oncology, vol. 8, no. 2, p. 101124, 2023.
@article{saade2023ultrahigh,
title = {Ultrahigh-Dose-Rate Proton Irradiation Elicits Reduced Toxicity in Zebrafish Embryos},
author = {Gaëlle Saade and Eva Bogaerts and Sophie Chiavassa and Guillaume Blain and Grégory Delpon and Manon Evin and Youssef Ghannam and Ferid Haddad and Karin Haustermans and Charbel Koumeir and others},
url = {https://www.sciencedirect.com/science/article/pii/S2452109422002305},
doi = {10.1016/j.adro.2022.101124},
year = {2023},
date = {2023-03-01},
urldate = {2023-03-01},
journal = {Advances in Radiation Oncology},
volume = {8},
number = {2},
pages = {101124},
publisher = {Elsevier},
abstract = {Purpose
Recently, ultrahigh-dose-rate radiation therapy (UHDR-RT) has emerged as a promising strategy to increase the benefit/risk ratio of external RT. Extensive work is on the way to characterize the physical and biological parameters that control the so-called “Flash” effect. However, this healthy/tumor differential effect is observable in in vivo models, which thereby drastically limits the amount of work that is achievable in a timely manner.
Methods and Materials
In this study, zebrafish embryos were used to compare the effect of UHDR irradiation (8-9 kGy/s) to conventional RT dose rate (0.2 Gy/s) with a 68 MeV proton beam. Viability, body length, spine curvature, and pericardial edema were measured 4 days postirradiation.
Results
We show that body length is significantly greater after UHDR-RT compared with conventional RT by 180 µm at 30 Gy and 90 µm at 40 Gy, while pericardial edema is only reduced at 30 Gy. No differences were obtained in terms of survival or spine curvature.
Conclusions
Zebrafish embryo length appears as a robust endpoint, and we anticipate that this model will substantially fasten the study of UHDR proton-beam parameters necessary for “Flash.”},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Recently, ultrahigh-dose-rate radiation therapy (UHDR-RT) has emerged as a promising strategy to increase the benefit/risk ratio of external RT. Extensive work is on the way to characterize the physical and biological parameters that control the so-called “Flash” effect. However, this healthy/tumor differential effect is observable in in vivo models, which thereby drastically limits the amount of work that is achievable in a timely manner.
Methods and Materials
In this study, zebrafish embryos were used to compare the effect of UHDR irradiation (8-9 kGy/s) to conventional RT dose rate (0.2 Gy/s) with a 68 MeV proton beam. Viability, body length, spine curvature, and pericardial edema were measured 4 days postirradiation.
Results
We show that body length is significantly greater after UHDR-RT compared with conventional RT by 180 µm at 30 Gy and 90 µm at 40 Gy, while pericardial edema is only reduced at 30 Gy. No differences were obtained in terms of survival or spine curvature.
Conclusions
Zebrafish embryo length appears as a robust endpoint, and we anticipate that this model will substantially fasten the study of UHDR proton-beam parameters necessary for “Flash.”
Nifontova, Galina; Petrova, Irina; Gerasimovich, Evgeniia; Konopsky, Valery N.; Ayadi, Nizar; Charlier, Cathy; Fleury, Fabrice; Karaulov, Alexander; Sukhanova, Alyona; Nabiev, Igor
Label-Free Multiplexed Microfluidic Analysis of Protein Interactions Based on Photonic Crystal Surface Mode Imaging Article de journal
Dans: International Journal of Molecular Sciences, vol. 24, no. 5, 2023, ISSN: 1422-0067.
@article{ijms24054347b,
title = {Label-Free Multiplexed Microfluidic Analysis of Protein Interactions Based on Photonic Crystal Surface Mode Imaging},
author = {Galina Nifontova and Irina Petrova and Evgeniia Gerasimovich and Valery N. Konopsky and Nizar Ayadi and Cathy Charlier and Fabrice Fleury and Alexander Karaulov and Alyona Sukhanova and Igor Nabiev},
url = {https://www.mdpi.com/1422-0067/24/5/4347},
doi = {10.3390/ijms24054347},
issn = {1422-0067},
year = {2023},
date = {2023-02-22},
urldate = {2023-02-22},
journal = {International Journal of Molecular Sciences},
volume = {24},
number = {5},
abstract = {High-throughput protein assays are crucial for modern diagnostics, drug discovery, proteomics, and other fields of biology and medicine. It allows simultaneous detection of hundreds of analytes and miniaturization of both fabrication and analytical procedures. Photonic crystal surface mode (PC SM) imaging is an effective alternative to surface plasmon resonance (SPR) imaging used in conventional gold-coated, label-free biosensors. PC SM imaging is advantageous as a quick, label-free, and reproducible technique for multiplexed analysis of biomolecular interactions. PC SM sensors are characterized by a longer signal propagation at the cost of a lower spatial resolution, which makes them more sensitive than classical SPR imaging sensors. We describe an approach for designing label-free protein biosensing assays employing PC SM imaging in the microfluidic mode. Label-free, real-time detection of PC SM imaging biosensors using two-dimensional imaging of binding events has been designed to study arrays of model proteins (antibodies, immunoglobulin G-binding proteins, serum proteins, and DNA repair proteins) at 96 points prepared by automated spotting. The data prove feasibility of simultaneous PC SM imaging of multiple protein interactions. The results pave the way to further develop PC SM imaging as an advanced label-free microfluidic assay for the multiplexed detection of protein interactions.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Madel, Maria-Bernadette; Halper, Julia; Ibáñez, Lidia; Claire, Lozano; Rouleau, Matthieu; Boutin, Antoine; Mahler, Adrien; Pontier-Bres, Rodolphe; Ciucci, Thomas; Topi, Majlinda; Hue, Christophe; Amiaud, Jerome; Iborra, Salvador; Sancho, David; Heymann, Dominique; Garchon, Henri-Jean; Czerucka, Dorota; Apparailly, Florence; Duroux-Richard, Isabelle; Wakkach, Abdelilah; Blin-Wakkach, Claudine
Specific targeting of inflammatory osteoclastogenesis by the probiotic yeast S. boulardii CNCM I-745 reduces bone loss in osteoporosis Article de journal
Dans: eLife, vol. 12, p. e82037, 2023, ISSN: 2050-084X.
@article{10.7554/eLife.82037,
title = {Specific targeting of inflammatory osteoclastogenesis by the probiotic yeast S. boulardii CNCM I-745 reduces bone loss in osteoporosis},
author = {Maria-Bernadette Madel and Julia Halper and Lidia Ibáñez and Lozano Claire and Matthieu Rouleau and Antoine Boutin and Adrien Mahler and Rodolphe Pontier-Bres and Thomas Ciucci and Majlinda Topi and Christophe Hue and Jerome Amiaud and Salvador Iborra and David Sancho and Dominique Heymann and Henri-Jean Garchon and Dorota Czerucka and Florence Apparailly and Isabelle Duroux-Richard and Abdelilah Wakkach and Claudine Blin-Wakkach},
editor = {Yi-Ping Li and Mone Zaidi and Marco Ponzetti},
url = {https://doi.org/10.7554/eLife.82037},
doi = {10.7554/eLife.82037},
issn = {2050-084X},
year = {2023},
date = {2023-02-01},
urldate = {2023-02-01},
journal = {eLife},
volume = {12},
pages = {e82037},
publisher = {eLife Sciences Publications, Ltd},
abstract = {Bone destruction is a hallmark of chronic inflammation, and bone-resorbing osteoclasts arising under such a condition differ from steady-state ones. However, osteoclast diversity remains poorly explored. Here, we combined transcriptomic profiling, differentiation assays and in vivo analysis in mouse to decipher specific traits for inflammatory and steady-state osteoclasts. We identified and validated the pattern-recognition receptors (PRR) Tlr2, Dectin-1, and Mincle, all involved in yeast recognition as major regulators of inflammatory osteoclasts. We showed that administration of the yeast probiotic textitSaccharomyces boulardii CNCM I-745 (textitSb) in vivo reduced bone loss in ovariectomized but not sham mice by reducing inflammatory osteoclastogenesis. This beneficial impact of textitSb is mediated by the regulation of the inflammatory environment required for the generation of inflammatory osteoclasts. We also showed that textitSb derivatives as well as agonists of Tlr2, Dectin-1, and Mincle specifically inhibited directly the differentiation of inflammatory but not steady-state osteoclasts in vitro. These findings demonstrate a preferential use of the PRR-associated costimulatory differentiation pathway by inflammatory osteoclasts, thus enabling their specific inhibition, which opens new therapeutic perspectives for inflammatory bone loss.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Oliver, Lisa; Álvarez-Arenas, Arturo; Salaud, Céline; Jiménez-Sanchez, Juan; Calvo, Gabriel F.; Belmonte-Beitia, Juan; Blandin, Stephanie; Vidal, Luciano; Pérez, Victor; Heymann, Dominique; Vallette, François M.
A Simple 3D Cell Culture Method for Studying the Interactions between Human Mesenchymal Stromal/Stem Cells and Patients Derived Glioblastoma Article de journal
Dans: Cancers, vol. 15, no. 4, 2023, ISSN: 2072-6694.
@article{cancers15041304,
title = {A Simple 3D Cell Culture Method for Studying the Interactions between Human Mesenchymal Stromal/Stem Cells and Patients Derived Glioblastoma},
author = {Lisa Oliver and Arturo Álvarez-Arenas and Céline Salaud and Juan Jiménez-Sanchez and Gabriel F. Calvo and Juan Belmonte-Beitia and Stephanie Blandin and Luciano Vidal and Victor Pérez and Dominique Heymann and François M. Vallette},
url = {https://www.mdpi.com/2072-6694/15/4/1304},
doi = {10.3390/cancers15041304},
issn = {2072-6694},
year = {2023},
date = {2023-01-01},
urldate = {2023-01-01},
journal = {Cancers},
volume = {15},
number = {4},
abstract = {We have developed a 3D biosphere model using patient-derived cells (PDCs) from glioblastoma (GBM), the major form of primary brain tumors in adult, plus cancer-activated fibroblasts (CAFs), obtained by culturing mesenchymal stem cells with GBM conditioned media. The effect of MSC/CAFs on the proliferation, cell-cell interactions, and response to treatment of PDCs was evaluated. Proliferation in the presence of CAFs was statistically lower but the spheroids formed within the 3D-biosphere were larger. A treatment for 5 days with Temozolomide (TMZ) and irradiation, the standard therapy for GBM, had a marked effect on cell number in monocultures compared to co-cultures and influenced cancer stem cells composition, similar to that observed in GBM patients. Mathematical analyses of spheroids growth and morphology confirm the similarity with GBM patients. We, thus, provide a simple and reproducible method to obtain 3D cultures from patient-derived biopsies and co-cultures with MSC with a near 100% success. This method provides the basis for relevant in vitro functional models for a better comprehension of the role of tumor microenvironment and, for precision and/or personalized medicine, potentially to predict the response to treatments for each GBM patient.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Jacquot, Perrine; Muñoz-Garcia, Javier; Fleury, Maurine; Cochonneau, Denis; Gaussin, Rémi; Enouf, Elise; Roze, Caroline; Ollivier, Emilie; Cinier, Mathieu; Heymann, Dominique
Engineering of a Bispecific Nanofitin with Immune Checkpoint Inhibitory Activity Conditioned by the Cross-Arm Binding to EGFR and PDL1 Article de journal
Dans: Biomolecules, vol. 13, no. 4, 2023, ISSN: 2218-273X.
@article{biom13040636,
title = {Engineering of a Bispecific Nanofitin with Immune Checkpoint Inhibitory Activity Conditioned by the Cross-Arm Binding to EGFR and PDL1},
author = {Perrine Jacquot and Javier Muñoz-Garcia and Maurine Fleury and Denis Cochonneau and Rémi Gaussin and Elise Enouf and Caroline Roze and Emilie Ollivier and Mathieu Cinier and Dominique Heymann},
url = {https://www.mdpi.com/2218-273X/13/4/636},
doi = {10.3390/biom13040636},
issn = {2218-273X},
year = {2023},
date = {2023-01-01},
urldate = {2023-01-01},
journal = {Biomolecules},
volume = {13},
number = {4},
abstract = {Re-education of the tumor microenvironment with immune checkpoint inhibitors (ICI) has provided the most significant advancement in cancer management, with impressive efficacy and durable response reported. However, low response rates and a high frequency of immune-related adverse events (irAEs) remain associated with ICI therapies. The latter can be linked to their high affinity and avidity for their target that fosters on-target/off-tumor binding and subsequent breaking of immune self-tolerance in normal tissues. Many multispecific protein formats have been proposed to increase the tumor cell's selectivity of ICI therapies. In this study, we explored the engineering of a bispecific Nanofitin by the fusion of an anti-epidermal growth factor receptor (EGFR) and anti-programmed cell death ligand 1 (PDL1) Nanofitin modules. While lowering the affinity of the Nanofitin modules for their respective target, the fusion enables the simultaneous engagement of EGFR and PDL1, which translates into a selective binding to tumor cells co-expressing EGFR and PDL1 only. We demonstrated that affinity-attenuated bispecific Nanofitin could elicit PDL1 blockade exclusively in an EGFR-directed manner. Overall, the data collected highlight the potential of this approach to enhance the selectivity and safety of PDL1 checkpoint inhibition.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
13 publications
CHALOPIN, Antoine
2022.
@mastersthesis{chalopin2022,
title = {Caractérisation des cellules tumorales circulantes de sarcomes osseux : identification de nouveaux marqueurs de la pathologie recidivante},
author = {Antoine CHALOPIN},
url = {https://theses.hal.science/tel-03937416},
year = {2022},
date = {2022-12-20},
urldate = {2022-12-20},
keywords = {},
pubstate = {published},
tppubtype = {mastersthesis}
}
Beird, Hannah C; Bielack, Stefan S; Flanagan, Adrienne M; Gill, Jonathan; Heymann, Dominique; Janeway, Katherine A; Livingston, J Andrew; Roberts, Ryan D; Strauss, Sandra J; Gorlick, Richard
Osteosarcoma Article de journal
Dans: Nat Rev Dis Primers, vol. 8, no. 1, p. 77, 2022, ISSN: 2056-676X.
@article{pmid36481668,
title = {Osteosarcoma},
author = {Hannah C Beird and Stefan S Bielack and Adrienne M Flanagan and Jonathan Gill and Dominique Heymann and Katherine A Janeway and J Andrew Livingston and Ryan D Roberts and Sandra J Strauss and Richard Gorlick},
doi = {10.1038/s41572-022-00409-y},
issn = {2056-676X},
year = {2022},
date = {2022-12-01},
urldate = {2022-12-01},
journal = {Nat Rev Dis Primers},
volume = {8},
number = {1},
pages = {77},
abstract = {Osteosarcoma is the most common primary malignant tumour of the bone. Osteosarcoma incidence is bimodal, peaking at 18 and 60 years of age, and is slightly more common in males. The key pathophysiological mechanism involves several possible genetic drivers of disease linked to bone formation, causing malignant progression and metastasis. While there have been significant improvements in the outcome of patients with localized disease, with event-free survival outcomes exceeding 60%, in patients with metastatic disease, event-free survival outcomes remain poor at less than 30%. The suspicion of osteosarcoma based on radiographs still requires pathological evaluation of a bone biopsy specimen for definitive diagnosis and CT imaging of the chest should be performed to identify lung nodules. So far, population-based screening and surveillance strategies have not been implemented due to the rarity of osteosarcoma and the lack of reliable markers. Current screening focuses only on groups at high risk such as patients with genetic cancer predisposition syndromes. Management of osteosarcoma requires a multidisciplinary team of paediatric and medical oncologists, orthopaedic and general surgeons, pathologists, radiologists and specialist nurses. Survivors of osteosarcoma require specialized medical follow-up, as curative treatment consisting of chemotherapy and surgery has long-term adverse effects, which also affect the quality of life of patients. The development of osteosarcoma model systems and related research as well as the evaluation of new treatment approaches are ongoing to improve disease outcomes, especially for patients with metastases.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Potiron, V; Delpon, G; Ollivier, L; Vaugier, L; Doré, M; Guimas, V; Rio, E; Thillays, F; Llagostera, C; Moignier, A; Josset, S; Chiavassa, S; Perennec, T; Supiot, S
[Clinical research in radiation oncology: how to move from the laboratory to the patient?] Article de journal
Dans: Cancer Radiother, vol. 26, no. 6-7, p. 808–813, 2022, ISSN: 1769-6658.
@article{pmid35999162b,
title = {[Clinical research in radiation oncology: how to move from the laboratory to the patient?]},
author = {V Potiron and G Delpon and L Ollivier and L Vaugier and M Doré and V Guimas and E Rio and F Thillays and C Llagostera and A Moignier and S Josset and S Chiavassa and T Perennec and S Supiot},
doi = {10.1016/j.canrad.2022.07.009},
issn = {1769-6658},
year = {2022},
date = {2022-10-01},
urldate = {2022-10-01},
journal = {Cancer Radiother},
volume = {26},
number = {6-7},
pages = {808--813},
abstract = {Translational research in radiation oncology is undergoing intense development. An increasingly rapid transfer is taking place from the laboratory to the patients, both in the selection of patients who can benefit from radiotherapy and in the development of innovative irradiation strategies or the development of combinations with drugs. Accelerating the passage of discoveries from the laboratory to the clinic represents the ideal of any translational research program but requires taking into account the multiple obstacles that can slow this progress. The ambition of the RadioTransNet network, a project to structure preclinical research in radiation oncology in France, is precisely to promote scientific and clinical interactions at the interface of radiotherapy and radiobiology, in its preclinical positioning, in order to identify priorities for strategic research dedicated to innovation in radiotherapy. The multidisciplinary radiotherapy teams with experts in biology, medicine, medical physics, mathematics and engineering sciences are able to meet these new challenges which will allow these advances to be made available to patients as quickly as possible.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Heymann, Clément J F; Bobin-Dubigeon, Christine; Muñoz-Garcia, Javier; Cochonneau, Denis; Ollivier, Emilie; Heymann, Marie-Françoise; Heymann, Dominique
Lipopolysaccharide-binding protein expression is associated to the metastatic status of osteosarcoma patients Article de journal
Dans: J Bone Oncol, vol. 36, p. 100451, 2022, ISSN: 2212-1366.
@article{pmid35990515,
title = {Lipopolysaccharide-binding protein expression is associated to the metastatic status of osteosarcoma patients},
author = {Clément J F Heymann and Christine Bobin-Dubigeon and Javier Muñoz-Garcia and Denis Cochonneau and Emilie Ollivier and Marie-Françoise Heymann and Dominique Heymann},
doi = {10.1016/j.jbo.2022.100451},
issn = {2212-1366},
year = {2022},
date = {2022-10-01},
urldate = {2022-10-01},
journal = {J Bone Oncol},
volume = {36},
pages = {100451},
abstract = {Osteosarcoma (OS) is a rare malignant primary bone tumours characterized by a high genetic and cell composition heterogeneity. Unfortunately, despite the use of drug combinations and the recent development of immunotherapies, the overall survival has not improved in the last four decades. Due to the key role of the tumour microenvironment in the pathogenesis of OS, a better understanding of its microenvironment is mandatory to develop new therapeutic approaches. From retrospective biological cohorts of OS, we analysed by immunohistochemistry the presence of lipopolysaccharide (LPS)-binding protein (LBP) in diagnostic biopsies with local disease and compared their level of infiltration to patients suffering from metastatic status. LBP is considered as a marker of LPS exposure and can indirectly reflect the presence of Gram-negative microbiota. LBP were detected in the cytoplasm of OS cells as well as in tumour-associated macrophage. Tumour samples of patients with local disease were significantly enriched in LBP compared to tumour tissues of patients with metastatic status. Lung metastatic tissues showed similar level of LBP compared to paired primary tumours. Overall, this study strongly suggests the presence of Gram-negative bacteria in OS tissues and demonstrated their significant differential level according the metastatic status. This tumour-associated microbiome may help in the conceptualisation of new therapeutic approach to trigger efficient therapeutic responses against cancer.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Rodríguez-Pena, Alejandro; Armendariz, Estibaliz; Oyarbide, Alvaro; Morales, Xabier; Ortiz-Espinosa, Sergio; de Córdoba, Borja Ruiz-Fernández; Cochonneau, Denis; Cornago, Iñaki; Heymann, Dominique; Argemi, Josepmaría; D'Avola, Delia; Sangro, Bruno; Lecanda, Fernando; Pio, Ruben; Cortés-Domínguez, Iván; Ortiz-de-Solórzano, Carlos
Design and validation of a tunable inertial microfluidic system for the efficient enrichment of circulating tumor cells in blood Article de journal
Dans: Bioeng Transl Med, vol. 7, no. 3, p. e10331, 2022, ISSN: 2380-6761.
@article{pmid36176621,
title = {Design and validation of a tunable inertial microfluidic system for the efficient enrichment of circulating tumor cells in blood},
author = {Alejandro Rodríguez-Pena and Estibaliz Armendariz and Alvaro Oyarbide and Xabier Morales and Sergio Ortiz-Espinosa and Borja Ruiz-Fernández de Córdoba and Denis Cochonneau and Iñaki Cornago and Dominique Heymann and Josepmaría Argemi and Delia D'Avola and Bruno Sangro and Fernando Lecanda and Ruben Pio and Iván Cortés-Domínguez and Carlos Ortiz-de-Solórzano},
doi = {10.1002/btm2.10331},
issn = {2380-6761},
year = {2022},
date = {2022-09-01},
urldate = {2022-09-01},
journal = {Bioeng Transl Med},
volume = {7},
number = {3},
pages = {e10331},
abstract = {The analysis of circulating tumor cells (CTCs) in blood is a powerful noninvasive alternative to conventional tumor biopsy. Inertial-based separation is a promising high-throughput, marker-free sorting strategy for the enrichment and isolation of CTCs. Here, we present and validate a double spiral microfluidic device that efficiently isolates CTCs with a fine-tunable cut-off value of 9 μm and a separation range of 2 μm. We designed the device based on computer simulations that introduce a novel, customized inertial force term, and provide practical fabrication guidelines. We validated the device using calibration beads, which allowed us to refine the simulations and redesign the device. Then we validated the redesigned device using blood samples and a murine model of metastatic breast cancer. Finally, as a proof of principle, we tested the device using peripheral blood from a patient with hepatocellular carcinoma, isolating more than 17 CTCs/ml, with purity/removal values of 96.03% and 99.99% of white blood cell and red blood cells, respectively. These results confirm highly efficient CTC isolation with a stringent cut-off value and better separation results than the state of the art.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Jubelin, Camille; Muñoz-Garcia, Javier; Griscom, Laurent; Cochonneau, Denis; Ollivier, Emilie; Heymann, Marie-Françoise; Vallette, François M; Oliver, Lisa; Heymann, Dominique
Three-dimensional in vitro culture models in oncology research Article de journal
Dans: Cell Biosci, vol. 12, no. 1, p. 155, 2022, ISSN: 2045-3701.
@article{pmid36089610,
title = {Three-dimensional in vitro culture models in oncology research},
author = {Camille Jubelin and Javier Muñoz-Garcia and Laurent Griscom and Denis Cochonneau and Emilie Ollivier and Marie-Françoise Heymann and François M Vallette and Lisa Oliver and Dominique Heymann},
doi = {10.1186/s13578-022-00887-3},
issn = {2045-3701},
year = {2022},
date = {2022-09-01},
urldate = {2022-09-01},
journal = {Cell Biosci},
volume = {12},
number = {1},
pages = {155},
abstract = {Cancer is a multifactorial disease that is responsible for 10 million deaths per year. The intra- and inter-heterogeneity of malignant tumors make it difficult to develop single targeted approaches. Similarly, their diversity requires various models to investigate the mechanisms involved in cancer initiation, progression, drug resistance and recurrence. Of the in vitro cell-based models, monolayer adherent (also known as 2D culture) cell cultures have been used for the longest time. However, it appears that they are often less appropriate than the three-dimensional (3D) cell culture approach for mimicking the biological behavior of tumor cells, in particular the mechanisms leading to therapeutic escape and drug resistance. Multicellular tumor spheroids are widely used to study cancers in 3D, and can be generated by a multiplicity of techniques, such as liquid-based and scaffold-based 3D cultures, microfluidics and bioprinting. Organoids are more complex 3D models than multicellular tumor spheroids because they are generated from stem cells isolated from patients and are considered as powerful tools to reproduce the disease development in vitro. The present review provides an overview of the various 3D culture models that have been set up to study cancer development and drug response. The advantages of 3D models compared to 2D cell cultures, the constraint, and the fields of application of these models and their techniques of production are also discussed.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Ollivier, Luc; Orione, Charles; Bore, Paul; Misery, Laurent; Legoupil, Delphine; Leclere, Jean-Christophe; Coste, Anne; Girault, Gilles; Sicard-Cras, Iona; Kacperek, Clemence; Lucia, Francois; Stefan, Dinu; Thillays, François; Rio, Emmanuel; Lesueur, Paul; Berthou, Christian; Heymann, Dominique; Champiat, Stéphane; Supiot, Stéphane; Vaugier, Loig; Kao, William
Abscopal Response in Metastatic Melanoma: Real-World Data of a Retrospective, Multicenter Study Article de journal
Dans: Cancers (Basel), vol. 14, no. 17, 2022, ISSN: 2072-6694.
@article{pmid36077747,
title = {Abscopal Response in Metastatic Melanoma: Real-World Data of a Retrospective, Multicenter Study},
author = {Luc Ollivier and Charles Orione and Paul Bore and Laurent Misery and Delphine Legoupil and Jean-Christophe Leclere and Anne Coste and Gilles Girault and Iona Sicard-Cras and Clemence Kacperek and Francois Lucia and Dinu Stefan and François Thillays and Emmanuel Rio and Paul Lesueur and Christian Berthou and Dominique Heymann and Stéphane Champiat and Stéphane Supiot and Loig Vaugier and William Kao},
doi = {10.3390/cancers14174213},
issn = {2072-6694},
year = {2022},
date = {2022-08-01},
urldate = {2022-08-01},
journal = {Cancers (Basel)},
volume = {14},
number = {17},
abstract = {OBJECTIVE: To evaluate the incidence of the abscopal response (AR) in patients with metastatic melanoma requiring palliative radiotherapy (RT).
PATIENTS AND METHODS: Patients treated for metastatic melanoma between January 1998 and February 2020 in four oncology departments were screened. Patients with progression under immune checkpoint inhibitors or without ongoing systemic treatment, and requiring palliative RT were considered. The AR was defined as an objective response according to RECIST and/or iRECIST for at least one non-irradiated metastasis at distance (≥10 cm) from the irradiated lesion. Primary endpoint was the rate of AR. Secondary endpoints were overall survival (OS), progression-free survival (PFS), local control (LC) of the irradiated lesion, and toxicity as assessed by CTCAE v5.
RESULTS: Over the period considered, 118 patients were included and analyzed. Fifteen patients (12.7%) had an AR. With a median follow-up of 7.7 months (range, 0.2-242.2), median OS and PFS after RT were significantly longer in patients with an AR compared to those without: 28 vs. 6.6 months ( < 0.01) and not reached vs. .2 months, respectively. No grade ≥2 toxicity was reported. Patients who developed an AR were more likely to be treated with immunotherapy (93.3% vs. 55.9%, 0.02). In multivariate analysis, they had a higher number of irradiated metastases treated concomitantly (HR = 16.9, < 0.01) and a higher rate of mild infections during RT (HR = 403.5, < 0.01).
CONCLUSIONS: AR in metastatic melanoma seems to be highly prognostic of overall survival, although it is a rare phenomenon. It may be promoted by multiple concomitant treatments with RT and immunotherapy and by acute inflammatory events such as infection.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
PATIENTS AND METHODS: Patients treated for metastatic melanoma between January 1998 and February 2020 in four oncology departments were screened. Patients with progression under immune checkpoint inhibitors or without ongoing systemic treatment, and requiring palliative RT were considered. The AR was defined as an objective response according to RECIST and/or iRECIST for at least one non-irradiated metastasis at distance (≥10 cm) from the irradiated lesion. Primary endpoint was the rate of AR. Secondary endpoints were overall survival (OS), progression-free survival (PFS), local control (LC) of the irradiated lesion, and toxicity as assessed by CTCAE v5.
RESULTS: Over the period considered, 118 patients were included and analyzed. Fifteen patients (12.7%) had an AR. With a median follow-up of 7.7 months (range, 0.2-242.2), median OS and PFS after RT were significantly longer in patients with an AR compared to those without: 28 vs. 6.6 months ( < 0.01) and not reached vs. .2 months, respectively. No grade ≥2 toxicity was reported. Patients who developed an AR were more likely to be treated with immunotherapy (93.3% vs. 55.9%, 0.02). In multivariate analysis, they had a higher number of irradiated metastases treated concomitantly (HR = 16.9, < 0.01) and a higher rate of mild infections during RT (HR = 403.5, < 0.01).
CONCLUSIONS: AR in metastatic melanoma seems to be highly prognostic of overall survival, although it is a rare phenomenon. It may be promoted by multiple concomitant treatments with RT and immunotherapy and by acute inflammatory events such as infection.
Jubelin, Camille; Cochonneau, Denis; Moranton, Emilie; Munoz-Garcia, Javier; Heymann, Dominique
Circulating Tumor Cells and ctDNA in Sarcomas Chapitre d'ouvrage
Dans: Leong, Stanley P.; Nathanson, S. David; Zager, Jonathan S. (Ed.): Cancer Metastasis Through the Lymphovascular System, p. 121–128, Springer, 2022.
@inbook{jubelin2022circulating,
title = {Circulating Tumor Cells and ctDNA in Sarcomas},
author = {Camille Jubelin and Denis Cochonneau and Emilie Moranton and Javier Munoz-Garcia and Dominique Heymann},
editor = {Stanley P. Leong and S. David Nathanson and Jonathan S. Zager},
doi = {10.1007/978-3-030-93084-4_12},
year = {2022},
date = {2022-06-25},
urldate = {2022-06-25},
booktitle = {Cancer Metastasis Through the Lymphovascular System},
pages = {121--128},
publisher = {Springer},
abstract = {Sarcomas are clustered in two oncological entities named bone and soft tissue sarcomas. Both are rare cancers originating from the mesenchyme, characterized by their propensity to induce the development of lung metastases. Sarcoma cells escaping from the primary tumor site spread to the pulmonary tissue through the bloodstream where they found a favorable microenvironment to establish metastatic foci. The low number of patients, the high histological, genetic, and molecular heterogeneity of sarcomas combined with the absence of specific markers expressed by cancer cells make the detection and follow-up of the minimal residual disease challenging. Over the last decade, tremendous technological progress has been made towards the detection of recurrent diseases. The literature is now enriched of information describing the use of liquid biopsies in clinical care of sarcoma patients. This chapter aims to give a brief overview of the most recent data available on the detection of circulating tumor cells and circulating tumor DNA in sarcomas.},
keywords = {},
pubstate = {published},
tppubtype = {inbook}
}
de Cordoba, Borja Ruiz-Fernandez; Moreno, Haritz; Valencia, Karmele; Perurena, Naiara; Ruedas, Pablo; Walle, Thomas; Pezonaga-Torres, Alberto; Hinojosa, Juan; Guruceaga, Elisabet; Pineda-Lucena, Antonio; Abengozar-Muela, Marta; Cochonneau, Denis; Zandueta, Carolina; Martinez-Canarias, Susana; Teijeira, Alvaro; Ajona, Daniel; Ortiz-Espinosa, Sergio; Morales, Xabier; de Solorzano, Carlos Ortiz; Santisteban, Marta; Ramos-Garcia, Luis I; Guembe, Laura; Strnad, Vratislav; Heymann, Dominique; Hervas-Stubbs, Sandra; Pio, Ruben; Rodriguez-Ruiz, Maria E; de Andrea, Carlos E; Vicent, Silvestre; Melero, Ignacio; Lecanda, Fernando; Martinez-Monge, Rafael
Tumor ENPP1(CD203a)/Haptoglobin Axis Exploits Myeloid-Derived Suppressor Cells to Promote Post-Radiotherapy Local Recurrence in Breast Cancer Article de journal
Dans: Cancer Discov, vol. 12, no. 5, p. 1356-1377, 2022, ISSN: 2159-8290.
@article{pmid35086922,
title = {Tumor ENPP1(CD203a)/Haptoglobin Axis Exploits Myeloid-Derived Suppressor Cells to Promote Post-Radiotherapy Local Recurrence in Breast Cancer},
author = {Borja Ruiz-Fernandez de Cordoba and Haritz Moreno and Karmele Valencia and Naiara Perurena and Pablo Ruedas and Thomas Walle and Alberto Pezonaga-Torres and Juan Hinojosa and Elisabet Guruceaga and Antonio Pineda-Lucena and Marta Abengozar-Muela and Denis Cochonneau and Carolina Zandueta and Susana Martinez-Canarias and Alvaro Teijeira and Daniel Ajona and Sergio Ortiz-Espinosa and Xabier Morales and Carlos Ortiz de Solorzano and Marta Santisteban and Luis I Ramos-Garcia and Laura Guembe and Vratislav Strnad and Dominique Heymann and Sandra Hervas-Stubbs and Ruben Pio and Maria E Rodriguez-Ruiz and Carlos E de Andrea and Silvestre Vicent and Ignacio Melero and Fernando Lecanda and Rafael Martinez-Monge},
url = {https://pubmed.ncbi.nlm.nih.gov/35086922/},
doi = {10.1158/2159-8290.CD-21-0932},
issn = {2159-8290},
year = {2022},
date = {2022-05-01},
urldate = {2022-05-01},
journal = {Cancer Discov},
volume = {12},
number = {5},
pages = {1356-1377},
abstract = {Locoregional failure (LRF) in breast cancer patients post-surgery and post-irradiation (IR) is linked to a dismal prognosis. In a refined new model, we identified Enpp1 (Ectonucleotide pyrophosphatase /phosphodiesterase 1/CD203a) to be closely associated with LRF. Enpp1high circulating tumor cells (CTC) contribute to relapse by a self-seeding mechanism. This process requires the infiltration of PMN-MDSC and neutrophil extracellular traps (NET) formation. Genetic and pharmacological Enpp1 inhibition or NET blockade extend relapse-free survival. Furthermore, in combination with fractionated irradiation (FD), Enpp1 abrogation obliterates LRF. Mechanistically, Enpp1-generated adenosinergic metabolites enhance Haptoglobin (Hp) expression. This inflammatory mediator elicits myeloid invasiveness and promotes NET formation. Accordingly, a significant increase in ENPP1 and NET formation is detected in relapsed human breast cancer tumors. Moreover, high ENPP1 or HP levels are associated with poor prognosis. These findings unveil the ENPP1/HP axis as an unanticipated mechanism exploited by tumor cells linking inflammation to immune remodeling favoring local relapse.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Muñoz-Garcia, Javier; Vargas-Franco, Jorge William; Royer, Bénédicte Brounais-Le; Cochonneau, Denis; Amiaud, Jérôme; Heymann, Marie-Françoise; Heymann, Dominique; Lézot, Frédéric
Inhibiting Endothelin Receptors with Macitentan Strengthens the Bone Protective Action of RANKL Inhibition and Reduces Metastatic Dissemination in Osteosarcoma Article de journal
Dans: Cancers, vol. 14, no. 7, p. 1765, 2022, ISSN: 2072-6694.
@article{cancers14071765,
title = {Inhibiting Endothelin Receptors with Macitentan Strengthens the Bone Protective Action of RANKL Inhibition and Reduces Metastatic Dissemination in Osteosarcoma},
author = {Javier Muñoz-Garcia and Jorge William Vargas-Franco and Bénédicte Brounais-Le Royer and Denis Cochonneau and Jérôme Amiaud and Marie-Françoise Heymann and Dominique Heymann and Frédéric Lézot},
url = {https://www.mdpi.com/2072-6694/14/7/1765},
doi = {10.3390/cancers14071765},
issn = {2072-6694},
year = {2022},
date = {2022-03-30},
urldate = {2022-01-01},
journal = {Cancers},
volume = {14},
number = {7},
pages = {1765},
abstract = {Current treatments for osteosarcoma, combining conventional polychemotherapy and surgery, make it possible to attain a five-year survival rate of 70% in affected individuals. The presence of chemoresistance and metastases significantly shorten the patient’s lifespan, making identification of new therapeutic tools essential. Inhibiting bone resorption has been shown to be an efficient adjuvant strategy impacting the metastatic dissemination of osteosarcoma, tumor growth, and associated bone destruction. Unfortunately, over-apposition of mineralized matrix by normal and tumoral osteoblasts was associated with this inhibition. Endothelin signaling is implicated in the functional differentiation of osteoblasts, raising the question of the potential value of inhibiting it alone, or in combination with bone resorption repression. Using mouse models of osteosarcoma, the impact of macitentan, an endothelin receptor inhibitor, was evaluated regarding tumor growth, metastatic dissemination, matrix over-apposition secondary to RANKL blockade, and safety when combined with chemotherapy. The results showed that macitentan has no impact on tumor growth or sensitivity to ifosfamide, but significantly reduces tumoral osteoid tissue formation and the metastatic capacity of the osteosarcoma. To conclude, macitentan appears to be a promising therapeutic adjuvant for osteosarcoma alone or associated with bone resorption inhibitors.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Jubelin, Camille; Munoz-Garcia, Javier; Cochonneau, Denis; Moranton, Emilie; Heymann, Marie Françoise; Heymann, Dominique
Biological evidence of cancer stem-like cells and recurrent disease in osteosarcoma Article de journal
Dans: Cancer Drug Resistance, vol. 5, iss. 5, p. 184-198, 2022.
@article{jubelin2022biological,
title = {Biological evidence of cancer stem-like cells and recurrent disease in osteosarcoma},
author = {Camille Jubelin and Javier Munoz-Garcia and Denis Cochonneau and Emilie Moranton and Marie Françoise Heymann and Dominique Heymann},
doi = {10.20517/cdr.2021.130},
year = {2022},
date = {2022-02-16},
urldate = {2022-02-16},
journal = {Cancer Drug Resistance},
volume = {5},
issue = {5},
pages = {184-198},
abstract = {Sarcomas are a large family of cancers originating in the mesenchyme. Composed of more than 100 histological subtypes, soft tissue and bone sarcomas remain clinically challenging, particularly in children and adolescents in whom sarcomas are the second most common malignant entities. Osteosarcoma is the main primary bone tumor in adolescents and young adults and is characterized by a high propensity to induce distant metastatic foci and become multi-drug resistant. The innate and acquired resistance of osteosarcoma can be explained by high histological heterogeneity and genetic/molecular diversity. In the last decade, the notion of cancer stem-like cells (CSCs) has emerged. This subset of cancer cells has been linked to drug resistance properties, recurrence of the disease, and therapeutic failure. Although CSCs remain controversial, many elements are in favor of them playing a role in the development of the drug resistance profile. The present review gives a brief overview of the most recent biological evidence of the presence of CSCs in osteosarcomas and their role in the drug resistance profile of these rare oncological entities. Their use as promising therapeutic targets is discussed.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Cadé, Marie; Muñoz-Garcia, Javier; Babuty, Antoine; Paré, Louis; Cochonneau, Denis; Fekir, Karim; Chatelais, Mathias; Heymann, Marie-Françoise; Lokajczyk, Anna; Boisson-Vidal, Catherine; Heymann, Dominique
FVIII regulates the molecular profile of endothelial cells: functional impact on the blood barrier and macrophage behavior Article de journal
Dans: Cell Mol Life Sci, vol. 79, no. 3, p. 145, 2022, ISSN: 1420-9071.
@article{pmid35190870,
title = {FVIII regulates the molecular profile of endothelial cells: functional impact on the blood barrier and macrophage behavior},
author = {Marie Cadé and Javier Muñoz-Garcia and Antoine Babuty and Louis Paré and Denis Cochonneau and Karim Fekir and Mathias Chatelais and Marie-Françoise Heymann and Anna Lokajczyk and Catherine Boisson-Vidal and Dominique Heymann},
doi = {10.1007/s00018-022-04178-5},
issn = {1420-9071},
year = {2022},
date = {2022-02-01},
urldate = {2022-02-01},
journal = {Cell Mol Life Sci},
volume = {79},
number = {3},
pages = {145},
abstract = {Hemophilia A is an inherited X-linked recessive bleeding disorder caused by deficient activity of blood coagulation factor VIII (FVIII). In addition, hemophilia patients show associated diseases including osteopenia, altered inflammation and vascular fragility which may represent the consequence of recurrent bleeding or may be related to the direct FVIII deficiency. Nowadays, recombinant FVIII is proposed to treat hemophilia patients with no circulating FVIII inhibitor. Initially described as a coenzyme to factor IXa for initiating thrombin generation, there is emerging evidence that FVIII is involved in multiple biological systems, including bone, vascular and immune systems. The present study investigated: (i) the functional activities of recombinant human FVIII (rFVIII) on endothelial cells, and (ii) the impact of rFVIII activities on the functional interactions of human monocytes and endothelial cells. We then investigated whether rFVIII had a direct effect on the adhesion of monocytes to the endothelium under physiological flow conditions. We observed that direct biological activities for rFVIII in endothelial cells were characterized by: (i) a decrease in endothelial cell adhesion to the underlying extracellular matrix; (ii) regulation of the transcriptomic and protein profiles of endothelial cells; (iii) an increase in the vascular tubes formed and vascular permeability in vitro; and (iv) an increase in monocyte adhesion activated endothelium and transendothelial migration. By regulating vascular permeability plus leukocyte adhesion and transendothelial migration, the present work highlights new biological functions for FVIII.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Colliec-Jouault, Sylvia; Sinquin, Corinne; Ratiskol, Jacqueline; Heymann, Dominique; Ruiz-Velasco, Carmen; Chesneau, Julie
Anti-metastatic marine bacterial exopolysaccharide derivative and uses thereof Patent
2022, (US Patent 11,219,638).
@patent{colliec2022anti,
title = {Anti-metastatic marine bacterial exopolysaccharide derivative and uses thereof},
author = {Sylvia Colliec-Jouault and Corinne Sinquin and Jacqueline Ratiskol and Dominique Heymann and Carmen Ruiz-Velasco and Julie Chesneau},
url = {https://patents.google.com/patent/US11219638B2/en},
year = {2022},
date = {2022-01-11},
urldate = {2022-01-11},
publisher = {Google Patents},
abstract = {The invention provides a low-molecular-weight (15 kDa) over-sulfated exopolysaccharide (GYS15) prepared from a marine native exopolysaccharide excreted by a mesophilic marine bacterium from a deep-sea hydrothermal environment, and relates to the use of this low-molecular-weight over-sulfated exopolysaccharide for the prevention or inhibition of metastases formation.},
note = {US Patent 11,219,638},
keywords = {},
pubstate = {published},
tppubtype = {patent}
}
4 publications
Velic, Denis; Demeyer, Alexandre; Peterlini, Thibaut; Benhelli-Mokrani, Houda; Mathé-Allainmat, Monique; Masson, Jean-Yves; Fleury, Fabrice
Molecular Determinant of DIDS Analogs Targeting RAD51 Activity Article de journal
Dans: Molecules, vol. 26, no. 18, p. 5460, 2021, ISSN: 1420-3049.
@article{velic_molecular_2021,
title = {Molecular Determinant of DIDS Analogs Targeting RAD51 Activity},
author = {Denis Velic and Alexandre Demeyer and Thibaut Peterlini and Houda Benhelli-Mokrani and Monique Mathé-Allainmat and Jean-Yves Masson and Fabrice Fleury},
url = {https://www.mdpi.com/1420-3049/26/18/5460},
doi = {10.3390/molecules26185460},
issn = {1420-3049},
year = {2021},
date = {2021-09-15},
urldate = {2021-09-15},
journal = {Molecules},
volume = {26},
number = {18},
pages = {5460},
abstract = {RAD51 is the central protein in DNA repair by homologous recombination (HR), involved in several steps of this process. It is shown that overexpression of the RAD51 protein is correlated with increased survival of cancer cells to cancer treatments. For the past decade, RAD51 overexpression-mediated resistance has justified the development of targeted inhibitors. One of the first molecules described to inhibit RAD51 was the 4,4 -diisothiocyanato-stilbene-2,2 -disulfonic acid (DIDS) molecule. This small molecule is effective in inhibiting different functions of RAD51, however its mode of action and the chemical functions involved in this inhibition have not been identified. In this work, we used several commercial molecules derived from DIDS to characterize the structural determinants involved in modulating the activity of RAD51. By combining biochemical and biophysical approaches, we have shown that DIDS and two analogs were able to inhibit the binding of RAD51 to ssDNA and prevent the formation of D-loop by RAD51. Both isothiocyanate substituents of DIDS appear to be essential in the inhibition of RAD51. These results open the way to the synthesis of new molecules derived from DIDS that should be greater modulators of RAD51 and more efficient for HR inhibition.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Demeyer, Alexandre; Benhelli-Mokrani, Houda; Chénais, B.; Weigel, Pierre; Fleury, Fabrice
Inhibiting homologous recombination by targeting RAD51 protein Article de journal
Dans: Biochimica et Biophysica Acta (BBA) - Reviews on Cancer, vol. 1876, no. 2, p. 188597, 2021, ISSN: 0304419X.
@article{demeyer_inhibiting_2021,
title = {Inhibiting homologous recombination by targeting RAD51 protein},
author = {Alexandre Demeyer and Houda Benhelli-Mokrani and B. Chénais and Pierre Weigel and Fabrice Fleury},
url = {https://linkinghub.elsevier.com/retrieve/pii/S0304419X21000949},
doi = {10.1016/j.bbcan.2021.188597},
issn = {0304419X},
year = {2021},
date = {2021-09-15},
urldate = {2021-09-15},
journal = {Biochimica et Biophysica Acta (BBA) - Reviews on Cancer},
volume = {1876},
number = {2},
pages = {188597},
abstract = {Homologous recombination (HR) is involved in repairing DNA double-strand breaks (DSB), the most harmful for the cell. Regulating HR is essential for maintaining genomic stability. In many forms of cancer, overactivation of HR increases tumor resistance to DNA-damaging treatments. RAD51, HR's core protein, is very often overexpressed in these cancers and plays a critical role in cancer cell development and survival. Targeting RAD51 directly to reduce its activity and its expression is therefore one strategy to sensitize and overcome resistance cancer cells to existing DNA-damaging therapies which remains the limiting factor for the success of targeted therapy.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Le, Van-Tuyen; Bertrand, Samuel; du Pont, Thibaut Robiou; Fleury, Fabrice; Caroff, Nathalie; Bourgeade-Delmas, Sandra; Gentil, Emmanuel; Logé, Cedric; Genta-Jouve, Gregory; Grovel, Olivier
Untargeted Metabolomics Approach for the Discovery of Environment-Related Pyran-2-Ones Chemodiversity in a Marine-Sourced Penicillium restrictum Article de journal
Dans: Marine Drugs, vol. 19, no. 7, p. 378, 2021, ISSN: 1660-3397.
@article{le_untargeted_2021,
title = {Untargeted Metabolomics Approach for the Discovery of Environment-Related Pyran-2-Ones Chemodiversity in a Marine-Sourced Penicillium restrictum},
author = {Van-Tuyen Le and Samuel Bertrand and Thibaut Robiou du Pont and Fabrice Fleury and Nathalie Caroff and Sandra Bourgeade-Delmas and Emmanuel Gentil and Cedric Logé and Gregory Genta-Jouve and Olivier Grovel},
url = {https://www.mdpi.com/1660-3397/19/7/378},
doi = {10.3390/md19070378},
issn = {1660-3397},
year = {2021},
date = {2021-09-15},
journal = {Marine Drugs},
volume = {19},
number = {7},
pages = {378},
abstract = {Very little is known about chemical interactions between fungi and their mollusc host within marine environments. Here, we investigated the metabolome of a Penicillium restrictum MMS417 strain isolated from the blue mussel Mytilus edulis collected on the Loire estuary, France. Following the OSMAC approach with the use of 14 culture media, the effect of salinity and of a musselderived medium on the metabolic expression were analysed using HPLC-UV/DAD-HRMS/MS. An untargeted metabolomics study was performed using principal component analysis (PCA), orthogonal projection to latent structure discriminant analysis (O-PLSDA) and molecular networking (MN). It highlighted some compounds belonging to sterols, macrolides and pyran-2-ones, which were specifically induced in marine conditions. In particular, a high chemical diversity of pyran-2-ones was found to be related to the presence of mussel extract in the culture medium. Mass spectrometry (MS)- and UV-guided purification resulted in the isolation of five new natural fungal pyran-2-one derivatives—5,6-dihydro-6S-hydroxymethyl-4-methoxy-2H-pyran-2-one (1), (6S, 1’R, 2’S)-LL-P880β (3), 5,6-dihydro-4-methoxy-6S-(1’S, 2’S-dihydroxy pent-3’(E)-enyl)-2H-pyran-2-one (4), 4-methoxy-6(1’R, 2’S-dihydroxy pent-3’(E)-enyl)-2H-pyran-2-one (6) and 4-methoxy-2H-pyran-2-one (7)—together with the known (6S, 1’S, 2’S)-LL-P880β (2), (1’R, 2’S)-LL-P880γ (5), 5,6-dihydro-4-methoxy-2H-pyran2-one (8), (6S, 1’S, 2’R)-LL-P880β (9), (6S, 1’S)-pestalotin (10), 1’R-dehydropestalotin (11) and 6-pentyl4-methoxy-2H-pyran-2-one (12) from the mussel-derived culture medium extract. The structures of 1-12 were determined by 1D- and 2D-MMR experiments as well as high-resolution tandem MS, ECD and DP4 calculations. Some of these compounds were evaluated for their cytotoxic, antibacterial, antileishmanial and in-silico PTP1B inhibitory activities. These results illustrate the utility in using host-derived media for the discovery of new natural products.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Muñoz-Garcia, Javier; Cochonneau, Denis; Télétchéa, Stéphane; Moranton, Emilie; Lanoe, Didier; Brion, Régis; Lézot, Frédéric; Heymann, Marie-Françoise; Heymann, Dominique
The twin cytokines interleukin-34 and CSF-1: masterful conductors of macrophage homeostasis Article de journal
Dans: Theranostics, vol. 11, no. 4, p. 1568–1593, 2021, ISSN: 1838-7640.
@article{pmid33408768,
title = {The twin cytokines interleukin-34 and CSF-1: masterful conductors of macrophage homeostasis},
author = {Javier Muñoz-Garcia and Denis Cochonneau and Stéphane Télétchéa and Emilie Moranton and Didier Lanoe and Régis Brion and Frédéric Lézot and Marie-Françoise Heymann and Dominique Heymann},
doi = {10.7150/thno.50683},
issn = {1838-7640},
year = {2021},
date = {2021-01-01},
urldate = {2021-01-01},
journal = {Theranostics},
volume = {11},
number = {4},
pages = {1568--1593},
abstract = {Macrophages are specialized cells that control tissue homeostasis. They include non-resident and tissue-resident macrophage populations which are characterized by the expression of particular cell surface markers and the secretion of molecules with a wide range of biological functions. The differentiation and polarization of macrophages relies on specific growth factors and their receptors. Macrophage-colony stimulating factor (CSF-1) and interleukine-34 (IL-34), also known as "twin" cytokines, are part of this regluatory landscape. CSF-1 and IL-34 share a common receptor, the macrophage-colony stimulating factor receptor (CSF-1R), which is activated in a similar way by both factors and turns on identical signaling pathways. However, there is some discrete differential activation leading to specific activities. In this review, we disscuss recent progress in understanding of the role of the twin cytokines in macrophage differentiation, from their interaction with CSF-1R and the activation of signaling pathways, to their implication in macrophage polarization of non-resident and tissue-resident macrophages. A special focus on IL-34, its involvement in pathophsyiological contexts, and its potential as a theranostic target for macrophage therapy will be proposed.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
10 publications
Chabot, Thomas; Cheraud, Yvonnick; Fleury, Fabrice
Relationships between DNA repair and RTK-mediated signaling pathways Article de journal
Dans: Biochimica et Biophysica Acta (BBA) - Reviews on Cancer, p. 188495, 2020, ISSN: 0304419X.
@article{Chabot2020,
title = {Relationships between DNA repair and RTK-mediated signaling pathways},
author = {Thomas Chabot and Yvonnick Cheraud and Fabrice Fleury},
url = {https://linkinghub.elsevier.com/retrieve/pii/S0304419X20302146},
doi = {10.1016/j.bbcan.2020.188495},
issn = {0304419X},
year = {2020},
date = {2020-12-01},
journal = {Biochimica et Biophysica Acta (BBA) - Reviews on Cancer},
pages = {188495},
publisher = {Elsevier},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Méresse, Sarah; Fodil, Mostefa; Fleury, Fabrice; Chénais, Benoît
Fucoxanthin, a Marine-Derived Carotenoid from Brown Seaweeds and Microalgae: A Promising Bioactive Compound for Cancer Therapy Article de journal
Dans: International Journal of Molecular Sciences, vol. 21, no. 23, p. 9273, 2020, ISSN: 1422-0067.
@article{Meresse2020,
title = {Fucoxanthin, a Marine-Derived Carotenoid from Brown Seaweeds and Microalgae: A Promising Bioactive Compound for Cancer Therapy},
author = {Sarah Méresse and Mostefa Fodil and Fabrice Fleury and Benoît Chénais},
url = {https://www.mdpi.com/1422-0067/21/23/9273},
doi = {10.3390/ijms21239273},
issn = {1422-0067},
year = {2020},
date = {2020-12-01},
journal = {International Journal of Molecular Sciences},
volume = {21},
number = {23},
pages = {9273},
publisher = {Multidisciplinary Digital Publishing Institute},
abstract = {Fucoxanthin is a well-known carotenoid of the xanthophyll family, mainly produced by marine organisms such as the macroalgae of the fucus genus or microalgae such as Phaeodactylum tricornutum. Fucoxanthin has antioxidant and anti-inflammatory properties but also several anticancer effects. Fucoxanthin induces cell growth arrest, apoptosis, and/or autophagy in several cancer cell lines as well as in animal models of cancer. Fucoxanthin treatment leads to the inhibition of metastasis-related migration, invasion, epithelial–mesenchymal transition, and angiogenesis. Fucoxanthin also affects the DNA repair pathways, which could be involved in the resistance phenotype of tumor cells. Moreover, combined treatments of fucoxanthin, or its metabolite fucoxanthinol, with usual anticancer treatments can support conventional therapeutic strategies by reducing drug resistance. This review focuses on the current knowledge of fucoxanthin with its potential anticancer properties, showing that fucoxanthin could be a promising compound for cancer therapy by acting on most of the classical hallmarks of tumor cells.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Chabot, Thomas
Université de Nantes, 2020, (294 pages).
@phdthesis{chabot2020modulation,
title = {Modulation de l'activité du Rad51 par le récepteur tyrosine kinase c-Met dans la réparation des cassures double-brin de l'ADN},
author = {Thomas Chabot},
url = {https://www.theses.fr/2020NANT1013},
year = {2020},
date = {2020-10-01},
school = {Université de Nantes},
abstract = {L'instabilité génomique due à la dérégulation des voies de réparation de l'ADN peut être à l’initiation de cancer et entraîner par la suite une résistance à la chimiothérapie et à la radiothérapie. La compréhension de ces mécanismes biologiques est donc essentielle dans la lutte contre le cancer. RAD51 est la protéine centrale de la voie de réparation des cassures double-brin de l'ADN par recombinaison homologue. Cette réparation conduit à une réparation fidèle de l'ADN. L'activité recombinase de la protéine RAD51 est finement régulée par des modifications post- traductionnelles telles que la phosphorylation. Au cours de la dernière décennie, de plus en plus d’études, suggèrent l'existence d'une relation entre les récepteurs à activité tyrosine kinases, souvent suractivés et impliqués dans l’agressivité et la prolifération cancéreuse, et la réparation de l'ADN. Parmi ces récepteurs à activité tyrosine kinases, le duo c-Met/HGF-SF est souvent muté, sur exprimé ou activé constitutivement dans de nombreux cancers et son inhibition a été montrée comme induisant une diminution de la réparation par recombinaison homologue. Au travers de cette thèse, nous montrons pour la première fois que c-Met est capable de phosphoryler la protéine RAD51 sur quatre résidus tyrosine localisés principalement dans l'interface monomère- monomère du nucléofilament de la recombinase humaine. Nous montrons l’implication de ces phosphorylations sur l’activité de RAD51 dans les différentes étapes de la recombinaison homologue. L'ensemble des résultats obtenus suggère le rôle possible de ces modifications dans la régulation de RAD51 et souligne l'importance de c-Met dans la réponse aux lésions de l'ADN.},
note = {294 pages},
keywords = {},
pubstate = {published},
tppubtype = {phdthesis}
}
Fleury, Fabrice; Demeyer, Alexandre; Weigel, Pierre; Chenais, Benoit; Mathé, Monique; Lebreton, Jacques
Disulfonate stilbenes for use in the treatment of proliferative diseases Patent
WO2020104634A1, 2020.
@patent{demeyer2020,
title = {Disulfonate stilbenes for use in the treatment of proliferative diseases},
author = {Fabrice Fleury and Alexandre Demeyer and Pierre Weigel and Benoit Chenais and Monique Mathé and Jacques Lebreton},
url = {https://worldwide.espacenet.com/patent/search/family/064564793/publication/WO2020104634A1?q=pn%3DWO2020104634A1},
year = {2020},
date = {2020-05-28},
number = {WO2020104634A1},
abstract = {This invention relates to compounds of general formula: wherein R0A and R0B are independently selected from hydrogen and pharmaceutically acceptable cations; and RA and RB are identical and selected from amide, carbamate, sulphonamide, azido, cyano and halide. The invention also relates to a pharmaceutical composition comprising a compound according to the invention. According to an embodiment, the composition further comprises another active ingredient, especially an antineoplastic agent. The invention also relates to a compound or a composition according to the invention for use as a medicament, especially a compound or a composition for use in the treatment of a proliferative disease such as for example cancer.},
keywords = {},
pubstate = {published},
tppubtype = {patent}
}
Ayadi, Nizar; Lafont, Florian; Charlier, Cathy; Benhelli-Mokrani, Houda; Sokolov, Pavel; Sukhanova, Alyona; Fleury, Fabrice; Nabiev, Igor
Comparative Advantages and Limitations of Quantum Dots in Protein Array Applications Chapitre d'ouvrage
Dans: Quantum Dots, vol. 2135, p. 259–273, Springer, New York, NY, Humana, 2020.
@inbook{cEQ3:ayadi_FLEURY:2020,
title = {Comparative Advantages and Limitations of Quantum Dots in Protein Array Applications},
author = {Nizar Ayadi and Florian Lafont and Cathy Charlier and Houda Benhelli-Mokrani and Pavel Sokolov and Alyona Sukhanova and Fabrice Fleury and Igor Nabiev},
year = {2020},
date = {2020-04-01},
booktitle = {Quantum Dots},
volume = {2135},
pages = {259--273},
publisher = {Springer},
address = {New York, NY},
edition = {Humana},
series = {Methods in Molecular Biology},
keywords = {},
pubstate = {published},
tppubtype = {inbook}
}
Yaremenko, Ivan A; Coghi, Paolo; Prommana, Parichat; Qiu, Congling; Radulov, Peter S; Qu, Yuanqing; Belyakova, Yulia Yu; Zanforlin, Enrico; Kokorekin, Vladimir A; Wu, Yuki Yu Jun; Fleury, Fabrice; Uthaipibull, Chairat; Wong, Vincent Kam Wai; Terent'ev, Alexander O
Synthetic Peroxides Promote Apoptosis of Cancer Cells by Inhibiting P-Glycoprotein ABCB5 Article de journal
Dans: ChemMedChem, vol. 15, no. 13, p. 1118–1127, 2020, ISSN: 18607187.
@article{Yaremenko2020b,
title = {Synthetic Peroxides Promote Apoptosis of Cancer Cells by Inhibiting P-Glycoprotein ABCB5},
author = {Ivan A Yaremenko and Paolo Coghi and Parichat Prommana and Congling Qiu and Peter S Radulov and Yuanqing Qu and Yulia Yu Belyakova and Enrico Zanforlin and Vladimir A Kokorekin and Yuki Yu Jun Wu and Fabrice Fleury and Chairat Uthaipibull and Vincent Kam Wai Wong and Alexander O Terent'ev},
doi = {10.1002/cmdc.202000042},
issn = {18607187},
year = {2020},
date = {2020-03-10},
journal = {ChemMedChem},
volume = {15},
number = {13},
pages = {1118--1127},
abstract = {This article discloses a new horizon for the application of peroxides in medical chemistry. Stable cyclic peroxides are demonstrated to have cytotoxic activity against cancer cells: in addition a mechanism of cytotoxic action is proposed. Synthetic bridged 1,2,4,5‐tetraoxanes and ozonides were effective against HepG2 cancer cells and some ozonides selectively targeted liver cancer cells (the selectivity indexes for compounds 11 b and 12 a are 8 and 5, respectively).
In some instances, tetraoxanes and ozonides were more selective than paclitaxel, artemisinin and artenusic acid.
Annexin V flow‐cytometry analysis revealed that the active ozonides 22 a and 23 a induced cell death of HepG2 by apoptosis. Further study showed that compounds 22 a and 23 a exhibited a strong inhibitory effect on P‐glycoprotein (P‐gp/ABCB5)‐overexpressing HepG2 cancer cells. ABCB5 is a key player in the multidrug‐resistant phenotype of liver cancer. Peroxides failed to demonstrate a direct correlation between oxidative potential and their biological activity. To our knowledge this is the first time that peroxide diastereoisomers have been found to show stereospecific antimalarial action against the chloroquine‐sensitive 3D7 strain of Plasmodium falciparum. Stereoisomeric ozonide 12 b is 11 times more active than stereoisomeric ozonide 12 a (IC50=5.81 vs 65.18 μm). Current findings mean that ozonides merit further investigation as potential therapeutic agents for drug‐resistant hepatocellular carcinoma.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
In some instances, tetraoxanes and ozonides were more selective than paclitaxel, artemisinin and artenusic acid.
Annexin V flow‐cytometry analysis revealed that the active ozonides 22 a and 23 a induced cell death of HepG2 by apoptosis. Further study showed that compounds 22 a and 23 a exhibited a strong inhibitory effect on P‐glycoprotein (P‐gp/ABCB5)‐overexpressing HepG2 cancer cells. ABCB5 is a key player in the multidrug‐resistant phenotype of liver cancer. Peroxides failed to demonstrate a direct correlation between oxidative potential and their biological activity. To our knowledge this is the first time that peroxide diastereoisomers have been found to show stereospecific antimalarial action against the chloroquine‐sensitive 3D7 strain of Plasmodium falciparum. Stereoisomeric ozonide 12 b is 11 times more active than stereoisomeric ozonide 12 a (IC50=5.81 vs 65.18 μm). Current findings mean that ozonides merit further investigation as potential therapeutic agents for drug‐resistant hepatocellular carcinoma.
Aganyants, Hovsep; Weigel, Pierre; Hovhannisyan, Yeranuhi; Lecocq, Michèle; Koloyan, Haykanush; Hambardzumyan, Artur; Hovsepyan, Anichka; Hallet, Jean Noël; Sakanyan, Vehary
Rational engineering of the substrate specificity of a thermostable d-hydantoinase (Dihydropyrimidinase) Article de journal
Dans: High-Throughput, vol. 9, no. 1, 2020, ISSN: 25715135.
@article{Aganyants2020,
title = {Rational engineering of the substrate specificity of a thermostable d-hydantoinase (Dihydropyrimidinase)},
author = {Hovsep Aganyants and Pierre Weigel and Yeranuhi Hovhannisyan and Michèle Lecocq and Haykanush Koloyan and Artur Hambardzumyan and Anichka Hovsepyan and Jean Noël Hallet and Vehary Sakanyan},
doi = {10.3390/ht9010005},
issn = {25715135},
year = {2020},
date = {2020-03-01},
journal = {High-Throughput},
volume = {9},
number = {1},
publisher = {MDPI AG},
abstract = {D-hydantoinases catalyze an enantioselective opening of 5-and 6-membered cyclic structures and therefore can be used for the production of optically pure precursors for biomedical applications. The thermostable D-hydantoinase from Geobacillus stearothermophilus ATCC 31783 is a manganese-dependent enzyme and exhibits low activity towards bulky hydantoin derivatives. Homology modeling with a known 3D structure (PDB code: 1K1D) allowed us to identify the amino acids to be mutated at the substrate binding site and in its immediate vicinity to modulate the substrate specificity. Both single and double substituted mutants were generated by site-directed mutagenesis at appropriate sites located inside and outside of the stereochemistry gate loops (SGL) involved in the substrate binding. Substrate specificity and kinetic constant data demonstrate that the replacement of Phe159 and Trp287 with alanine leads to an increase in the enzyme activity towards D,L-5-benzyl and D,L-5-indolylmethyl hydantoins. The length of the side chain and the hydrophobicity of substrates are essential parameters to consider when designing the substrate binding pocket for bulky hydantoins. Our data highlight that D-hydantoinase is the authentic dihydropyrimidinase involved in the pyrimidine reductive catabolic pathway in moderate thermophiles.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Yaremenko, Ivan A; Radulov, Peter S; Belyakova, Yulia Yu; Demina, Arina A; Fomenkov, Dmitriy I; Barsukov, Denis V; Subbotina, Irina R; Fleury, Fabrice; Terent'ev, Alexander O
Dans: Chemistry - A European Journal, vol. 26, no. 21, p. 4734–4751, 2020, ISSN: 15213765.
@article{Yaremenko2020a,
title = {Catalyst Development for the Synthesis of Ozonides and Tetraoxanes Under Heterogeneous Conditions: Disclosure of an Unprecedented Class of Fungicides for Agricultural Application},
author = {Ivan A Yaremenko and Peter S Radulov and Yulia Yu Belyakova and Arina A Demina and Dmitriy I Fomenkov and Denis V Barsukov and Irina R Subbotina and Fabrice Fleury and Alexander O Terent'ev},
doi = {10.1002/chem.201904555},
issn = {15213765},
year = {2020},
date = {2020-01-01},
journal = {Chemistry - A European Journal},
volume = {26},
number = {21},
pages = {4734--4751},
abstract = {The catalyst H3+xPMo12−x+6Mox+5O40 supported on SiO2 was developed for peroxidation of 1,3- and 1,5-diketones with hydrogen peroxide with the formation of bridged 1,2,4,5-tetraoxanes and bridged 1,2,4-trioxolanes (ozonides) with high yield based on isolated products (up to 86 and 90 %, respectively) under heterogeneous conditions. Synthesis of peroxides under heterogeneous conditions is a rare process and represents a challenge for this field of chemistry, because peroxides tend to decompose on the surface of a catalyst. A new class of antifungal agents for crop protection, that is, cyclic peroxides: bridged 1,2,4,5-tetraoxanes and bridged ozonides, was discovered. Some ozonides and tetraoxanes exhibit a very high antifungal activity and are superior to commercial fungicides, such as Triadimefon and Kresoxim-methyl. It is important to note that none of the fungicides used in agricultural chemistry contains a peroxide fragment.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Lafont, Florian; Fleury, Fabrice; Benhelli-Mokrani, Houda
DNA-PKcs Ser2056 auto-phosphorylation is affected by an O-GlcNAcylation/phosphorylation interplay Article de journal
Dans: Biochimica et Biophysica Acta (BBA) - General Subjects, vol. 1864, no. 12, p. 129705, 2020, ISSN: 0304-4165.
@article{LAFONT2020129705,
title = {DNA-PKcs Ser2056 auto-phosphorylation is affected by an O-GlcNAcylation/phosphorylation interplay},
author = {Florian Lafont and Fabrice Fleury and Houda Benhelli-Mokrani},
url = {http://www.sciencedirect.com/science/article/pii/S0304416520302178},
doi = {https://doi.org/10.1016/j.bbagen.2020.129705},
issn = {0304-4165},
year = {2020},
date = {2020-01-01},
journal = {Biochimica et Biophysica Acta (BBA) - General Subjects},
volume = {1864},
number = {12},
pages = {129705},
abstract = {Background DNA dependent Protein Kinase (DNA-PK) is an heterotrimeric complex regulating the Non Homologous End Joining (NHEJ) double strand break (DSB) repair pathway. The activity of its catalytic subunit (DNA-PKcs) is regulated by multiple phosphorylations, like the Ser2056 one that impacts DSB end processing and telomeres integrity. O-GlcNAcylation is a post translational modification (PTM) closely related to phosphorylation and its implication in the modulation of DNA-PKcs activity during the DNA Damage Response (DDR) is unknown. Methods Using IP techniques, and HeLa cell line, we evaluated the effect of pharmacological or siOGT mediated O-GlcNAc level modulation on DNA-PKcs O-GlcNAcylation. We used the RPA32 phosphorylation as a DNA-PKcs activity reporter substrate to evaluate the effect of O-GlcNAc modulators. Results We show here that human DNA-PKcs is an O-GlcNAc modified protein and that this new PTM is responsive to the cell O-GlcNAcylation level modulation. Our findings reveal that DNA-PKcs hypo O-GlcNAcylation affects its kinase activity and that the bleomycin-induced Ser2056 phosphorylation, is modulated by DNA-PKcs O-GlcNAcylation. Conclusions DNA-PKcs Ser2056 phosphorylation is antagonistically linked to DNA-PKcs O-GlcNAcylation level modulation. General significance Given the essential role of DNA-PKcs Ser2056 phosphorylation in the DDR, this study brings data about the role of cell O-GlcNAc level on genome integrity maintenance.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Vil', Vera A; Yaremenko, Ivan A; Fomenkov, Dmitri I; Levitsky, Dmitri O; Fleury, Fabrice; Terent'ev, Alexander O
Ion exchange resin-catalyzed synthesis of bridged tetraoxanes possessing in vitro cytotoxicity against HeLa cancer cells Article de journal
Dans: Chemistry of Heterocyclic Compounds, vol. 56, no. 6, p. 722–726, 2020, ISSN: 1573-8353.
@article{Vil2020,
title = {Ion exchange resin-catalyzed synthesis of bridged tetraoxanes possessing in vitro cytotoxicity against HeLa cancer cells},
author = {Vera A Vil' and Ivan A Yaremenko and Dmitri I Fomenkov and Dmitri O Levitsky and Fabrice Fleury and Alexander O Terent'ev},
url = {https://doi.org/10.1007/s10593-020-02722-4},
doi = {10.1007/s10593-020-02722-4},
issn = {1573-8353},
year = {2020},
date = {2020-01-01},
journal = {Chemistry of Heterocyclic Compounds},
volume = {56},
number = {6},
pages = {722--726},
abstract = {Bridged 1,2,4,5-tetraoxanes were prepared using available acidic ion exchange resin with high yields despite the possibility of peroxide decomposition under heterogeneous conditions. The bridged tetraoxanes demonstrated high cytotoxicity against HeLa cancer cells in vitro, which in some cases was higher than that of cisplatin, artesunate, and dihydroartemisinin.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2 publications
Velic, Denis; Charlier, Cathy; Popova, Milena; Jaunet-Lahary, Titouan; Bouchouireb, Zakaria; Henry, Sébastien; Weigel, Pierre; Masson, Jean-Yves; Laurent, Adèle D; Nabiev, Igor; Fleury, Fabrice
Interactions of the Rad51 inhibitor DIDS with human and bovine serum albumins: Optical spectroscopy and isothermal calorimetry approaches Article de journal
Dans: Biochimie, vol. 167, p. 187–197, 2019, ISSN: 0300-9084.
@article{VELIC2019187,
title = {Interactions of the Rad51 inhibitor DIDS with human and bovine serum albumins: Optical spectroscopy and isothermal calorimetry approaches},
author = {Denis Velic and Cathy Charlier and Milena Popova and Titouan Jaunet-Lahary and Zakaria Bouchouireb and Sébastien Henry and Pierre Weigel and Jean-Yves Masson and Adèle D Laurent and Igor Nabiev and Fabrice Fleury},
url = {http://www.sciencedirect.com/science/article/pii/S0300908419302743},
doi = {https://doi.org/10.1016/j.biochi.2019.09.016},
issn = {0300-9084},
year = {2019},
date = {2019-01-01},
journal = {Biochimie},
volume = {167},
pages = {187--197},
abstract = {Rad51 is a key protein in DNA repair by homologous recombination and an important target for development of drugs in cancer therapy. 4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS) has been used in clinic during the past 30 years as an inhibitor of anion transporters and channels. Recently DIDS has been demonstrated to affect Rad51-mediated homologous pairing and strand exchange, key processes in homologous recombination. Consequently, DIDS has been considered as a potential revertant of radio- and chemo-resistance of cancer cells, the major causes of therapy failure. Here, we have investigated the behavior of DIDS towards serum albumins. The effects of environmental factors, primarily, solvent polarity, on DIDS stability were evaluated, and the mechanisms of interaction of DIDS with human or bovine serum albumin were analyzed using isothermal calorimetry, circular dichroism and fluorescence spectroscopies. DIDS interaction with both serum albumins have been demonstrated, and the interaction characteristics have been determined. By comparing these characteristics for several DIDS derivatives, we have identified the DIDS moiety essential for the interaction. Furthermore, site competition data indicate that human albumin has two DIDS-binding sites: a high-affinity site in the IIIA subdomain and a low-affinity one in the IB subdomain. Molecular docking has revealed the key molecular moieties of DIDS responsible for its interactions in each site and shown that the IB site can bind two ligands. These findings show that binding of DIDS to serum albumin may change the balance between the free and bound DIDS forms, thereby affecting its bioavailability and efficacy against Rad51.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Chabot, Thomas; Defontaine, Alain; Marquis, Damien; Renodon-Corniere, Axelle; Courtois, Emmanuelle; Fleury, Fabrice; Cheraud, Yvonnick
New phosphorylation sites of rad51 by c-met modulates presynaptic filament stability Article de journal
Dans: Cancers, vol. 11, no. 3, 2019, ISSN: 20726694.
@article{Chabot2019a,
title = {New phosphorylation sites of rad51 by c-met modulates presynaptic filament stability},
author = {Thomas Chabot and Alain Defontaine and Damien Marquis and Axelle Renodon-Corniere and Emmanuelle Courtois and Fabrice Fleury and Yvonnick Cheraud},
doi = {10.3390/cancers11030413},
issn = {20726694},
year = {2019},
date = {2019-01-01},
journal = {Cancers},
volume = {11},
number = {3},
abstract = {Genomic instability through deregulation of DNA repair pathways can initiate cancer and subsequently result in resistance to chemo and radiotherapy. Understanding these biological mechanisms is therefore essential to overcome cancer. RAD51 is the central protein of the Homologous Recombination (HR) DNA repair pathway, which leads to faithful DNA repair of DSBs. The recombinase activity of RAD51 requires nucleofilament formation and is regulated by post-translational modifications such as phosphorylation. In the last decade, studies have suggested the existence of a relationship between receptor tyrosine kinases (RTK) and Homologous Recombination DNA repair. Among these RTK the c-MET receptor is often overexpressed or constitutively activated in many cancer types and its inhibition induces the decrease of HR. In this study, we show for the first time that c-MET is able to phosphorylate the RAD51 protein. We demonstrate in vitro that c-MET phosphorylates four tyrosine residues localized mainly in the subunit-subunit interface of RAD51. Whereas these post-translational modifications do not affect the presynaptic filament formation, they strengthen its stability against the inhibitor effect of the BRC peptide obtained from BRCA2. Taken together, these results confirm the role of these modifications in the regulation of the BRCA2-RAD51 interaction and underline the importance of c-MET in DNA damage response.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
4 publications
Benhelli-Mokrani, Houda; Mansuroglu, Zeyni; Chauderlier, Alban; Albaud, Benoit; Gentien, David; Sommer, Sabrina; Schirmer, Claire; Laqueuvre, Lucie; Josse, Thibaut; Buée, Luc; Lefebvre, Bruno; Galas, Marie Christine; Souès, Sylvie; Bonnefoy, Eliette
Genome-wide identification of genic and intergenic neuronal DNA regions bound by Tau protein under physiological and stress conditions Article de journal
Dans: Nucleic acids research, vol. 46, no. 21, p. 11405–11422, 2018, ISSN: 13624962.
@article{Benhelli-Mokrani2018,
title = {Genome-wide identification of genic and intergenic neuronal DNA regions bound by Tau protein under physiological and stress conditions},
author = {Houda Benhelli-Mokrani and Zeyni Mansuroglu and Alban Chauderlier and Benoit Albaud and David Gentien and Sabrina Sommer and Claire Schirmer and Lucie Laqueuvre and Thibaut Josse and Luc Buée and Bruno Lefebvre and Marie Christine Galas and Sylvie Sou{è}s and Eliette Bonnefoy},
doi = {10.1093/nar/gky929},
issn = {13624962},
year = {2018},
date = {2018-01-01},
journal = {Nucleic acids research},
volume = {46},
number = {21},
pages = {11405--11422},
abstract = {Tauopathies such as Alzheimer's Disease (AD) are neurodegenerative disorders for which there is presently no cure. They are named after the abnormal oligomerization/aggregation of the neuronal microtubule-associated Tau protein. Besides its role as a microtubule-associated protein, a DNA-binding capacity and a nuclear localization for Tau protein has been described in neurons. While questioning the potential role of Tau-DNA binding in the development of tauopathies, we have carried out a large-scale analysis of the interaction of Tau protein with the neuronal genome under physiological and heat stress conditions using the ChIP-on-chip technique that combines Chromatin ImmunoPrecipitation (ChIP) with DNA microarray (chip). Our findings show that Tau protein specifically interacts with genic and intergenic DNA sequences of primary culture of neurons with a preference for DNA regions positioned beyond the ±5000 bp range from transcription start site. An AG-rich DNA motif was found recurrently present within Tau-interacting regions and 30% of Tau-interacting regions overlapped DNA sequences coding for lncRNAs. Neurological processes affected in AD were enriched among Tau-interacting regions with in vivo gene expression assays being indicative of a transcriptional repressor role for Tau protein, which was exacerbated in neurons displaying nuclear pathological oligomerized forms of Tau protein.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Deriabin, Konstantin V; Yaremenko, Ivan A; Chislov, Mikhail V; Fleury, Fabrice; Terent'Ev, Alexander O; Islamova, Regina M
Similar nature leads to improved properties: Cyclic organosilicon triperoxides as promising curing agents for liquid polysiloxanes Article de journal
Dans: New Journal of Chemistry, vol. 42, no. 18, p. 15006–15013, 2018, ISSN: 13699261.
@article{Deriabin2018,
title = {Similar nature leads to improved properties: Cyclic organosilicon triperoxides as promising curing agents for liquid polysiloxanes},
author = {Konstantin V Deriabin and Ivan A Yaremenko and Mikhail V Chislov and Fabrice Fleury and Alexander O Terent'Ev and Regina M Islamova},
doi = {10.1039/c8nj02499e},
issn = {13699261},
year = {2018},
date = {2018-01-01},
journal = {New Journal of Chemistry},
volume = {42},
number = {18},
pages = {15006--15013},
publisher = {Royal Society of Chemistry},
abstract = {Cyclic organosilicon triperoxides were found to be vinyl-selective free-radical initiators for thermal curing at 100-180 °C of vinyl-terminated polydimethylsiloxane and trimethylsilyl-terminated polymethylhydrosiloxane producing homogeneous transparent silicone rubbers with antibacterial properties. The usage of the cyclic organosilicon triperoxides as the curing agents does not require free radical inhibitors in comparison with diacyl- and dialkyl peroxides. Among the tested compounds, the peroxide with the Me-Si-Me fragment and two cyclohexane rings is a much more active curing agent (180 °C},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Lafont, Florian; Ayadi, Nizar; Charlier, Cathy; Weigel, Pierre; Nabiev, Igor; Benhelli-Mokrani, Houda; Fleury, Fabrice
Assessment of DNA-PKcs kinase activity by quantum dot–based microarray Article de journal
Dans: Scientific Reports, vol. 8, no. 1, p. 1–12, 2018, ISSN: 20452322.
@article{Lafont2018,
title = {Assessment of DNA-PKcs kinase activity by quantum dot–based microarray},
author = {Florian Lafont and Nizar Ayadi and Cathy Charlier and Pierre Weigel and Igor Nabiev and Houda Benhelli-Mokrani and Fabrice Fleury},
doi = {10.1038/s41598-018-29256-2},
issn = {20452322},
year = {2018},
date = {2018-01-01},
journal = {Scientific Reports},
volume = {8},
number = {1},
pages = {1--12},
abstract = {Therapeutic efficacy against cancer is often based on a variety of DNA lesions, including DNA double-strand breaks (DSBs) which are repaired by homologous recombination and non-homologous end joining (NHEJ) pathways. In the past decade, the functions of the DNA repair proteins have been described as a potential mechanism of resistance in tumor cells. Therefore, the DNA repair proteins have become targets to improve the efficacy of anticancer therapy. Given the central role of DNA-PKcs in NHEJ, the therapeutic efficacy of targeting DNA-PKcs is frequently described as a strategy to prevent repair of treatment-induced DNA damage in cancer cells. The screening of a new inhibitor acting as a sensitizer requires the development of a high-throughput tool in order to identify and assess the most effective molecule. Here, we describe the elaboration of an antibody microarray dedicated to the NHEJ pathway that we used to evaluate the DNA-PKcs kinase activity in response to DNA damage. By combining a protein microarray with Quantum-Dot detection, we show that it is possible to follow the modification of phosphoproteomic cellular profiles induced by inhibitors during the response to DNA damage. Finally, we discuss the promising tool for screening kinase inhibitors and targeting DSB repair to improve cancer treatment.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Jaunet-Lahary, Titouan; Vercauteren, Daniel P; Fleury, Fabrice; Laurent, Adèle D
Computational simulations determining disulfonic stilbene derivative bioavailability within human serum albumin Article de journal
Dans: Physical Chemistry Chemical Physics, vol. 20, no. 26, p. 18020–18030, 2018, ISSN: 14639076.
@article{Jaunet-Lahary2018,
title = {Computational simulations determining disulfonic stilbene derivative bioavailability within human serum albumin},
author = {Titouan Jaunet-Lahary and Daniel P Vercauteren and Fabrice Fleury and Adèle D Laurent},
doi = {10.1039/c8cp00704g},
issn = {14639076},
year = {2018},
date = {2018-01-01},
journal = {Physical Chemistry Chemical Physics},
volume = {20},
number = {26},
pages = {18020--18030},
publisher = {Royal Society of Chemistry},
abstract = {Disulfonic stilbene (DS) derivatives are a member of the large family of compounds widely employed in medicine and biology as modulators for membrane transporters or inhibitors of a protein involved in DNA repair. They constitute interesting compounds that have not yet been investigated within the bioavailability framework. No crystallographic structures exist involving such compounds embedded in the most common drug carrier, human serum albumin (HSA). The present work studies, for the first time, the physico-chemical features driving the inclusion of three DS derivatives (amino, nitro and acetamido, named DADS, DNDS and DATDS, respectively) within the four common HSA binding sites using combined molecular docking and molecular dynamics simulations. A careful analysis of each ligand within each of the studied binding sites is carried out, highlighting specific interactions and key residues playing a role in stabilizing the ligand within each pocket. The comparison between DADS, DNDS and DATDS reveals that depending on the binding site, the conclusions are rather different. For instance, the IB binding site shows a specificity to DADS compounds while IIIA is the most favorable site for DNDS and DATDS. 2018},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
5 publications
Bosseboeuf, Adrien; Feron, Delphine; Tallet, Anne; Rossi, Cédric; Charlier, Cathy; Garderet, Laurent; Caillot, Denis; Moreau, Philippe; Cardó-Vila, Marina; Pasqualini, Renata; Arap, Wadih; Nelson, Alfreda Destea; Wilson, Bridget S; Perreault, Hélène; Piver, Eric; Weigel, Pierre; Girodon, François; Harb, Jean; Bigot-Corbel, Edith; Hermouet, Sylvie
Monoclonal IgG in MGUS and multiple myeloma targets infectious pathogens Article de journal
Dans: JCI Insight, vol. 2, no. 19, p. 1–18, 2017, ISSN: 0021-9738.
@article{Bosseboeuf2017,
title = {Monoclonal IgG in MGUS and multiple myeloma targets infectious pathogens},
author = {Adrien Bosseboeuf and Delphine Feron and Anne Tallet and Cédric Rossi and Cathy Charlier and Laurent Garderet and Denis Caillot and Philippe Moreau and Marina Cardó-Vila and Renata Pasqualini and Wadih Arap and Alfreda Destea Nelson and Bridget S Wilson and Hélène Perreault and Eric Piver and Pierre Weigel and François Girodon and Jean Harb and Edith Bigot-Corbel and Sylvie Hermouet},
doi = {10.1172/jci.insight.95367},
issn = {0021-9738},
year = {2017},
date = {2017-01-01},
journal = {JCI Insight},
volume = {2},
number = {19},
pages = {1--18},
abstract = {Subsets of mature B cell neoplasms are linked to infection with intracellular pathogens such as Epstein-Barr virus (EBV), hepatitis C virus (HCV), or Helicobacter pylori. However, the association between infection and the immunoglobulin-secreting (Ig-secreting) B proliferative disorders remains largely unresolved. We investigated whether the monoclonal IgG (mc IgG) produced by patients diagnosed with monoclonal gammopathy of undetermined significance (MGUS) or multiple myeloma (MM) targets infectious pathogens. Antigen specificity of purified mc IgG from a large patient cohort (n = 244) was determined using a multiplex infectious-antigen array (MIAA), which screens for reactivity to purified antigens or lysates from 9 pathogens. Purified mc IgG from 23.4% of patients (57 of 244) specifically recognized 1 pathogen in the MIAA. EBV was the most frequent target (15.6%), with 36 of 38 mc IgGs recognizing EBV nuclear antigen-1 (EBNA-1). MM patients with EBNA-1-specific mc IgG (14.0%) showed substantially greater bone marrow plasma cell infiltration and higher β2-microglobulin and inflammation/infection-linked cytokine levels compared with other smoldering myeloma/MM patients. Five other pathogens were the targets of mc IgG: herpes virus simplex-1 (2.9%), varicella zoster virus (1.6%), cytomegalovirus (0.8%), hepatitis C virus (1.2%), and H. pylori (1.2%). We conclude that a dysregulated immune response to infection may underlie disease onset and/or progression of MGUS and MM for subsets of patients.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Yaremenko, Ivan A; Syroeshkin, Mikhail A; Levitsky, Dmitri O; Fleury, Fabrice; Terent'ev, Alexander O
Cyclic peroxides as promising anticancer agents: in vitro cytotoxicity study of synthetic ozonides and tetraoxanes on human prostate cancer cell lines Article de journal
Dans: Medicinal Chemistry Research, vol. 26, no. 1, p. 170–179, 2017, ISSN: 1554-8120.
@article{Yaremenko2017,
title = {Cyclic peroxides as promising anticancer agents: in vitro cytotoxicity study of synthetic ozonides and tetraoxanes on human prostate cancer cell lines},
author = {Ivan A Yaremenko and Mikhail A Syroeshkin and Dmitri O Levitsky and Fabrice Fleury and Alexander O Terent'ev},
url = {https://doi.org/10.1007/s00044-016-1736-2},
doi = {10.1007/s00044-016-1736-2},
issn = {1554-8120},
year = {2017},
date = {2017-01-01},
journal = {Medicinal Chemistry Research},
volume = {26},
number = {1},
pages = {170--179},
abstract = {Synthetic ozonides and tetraoxanes were shown to have high cytotoxicity in vitro when tested on androgen-independent prostate cancer cell lines DU145 and PC3, which is in some cases was higher than that of doxorubicin, cisplatin, etoposide, artemisinin, and artesunate. Activity of ozonide stereoisomers differs from each other. This difference in activity and absence of correlation between activity of stereoisomers and their oxidative properties allow us to suggest existence of a quite specific mechanism of cytotoxicity of these endoperoxides different from a traditional mechanism based mainly on oxidative properties of peroxides.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Levitsky, Dmitri O; Gloriozova, Tatyana A; Poroikov, Vladimir V; Valery, M
ANABOLIC CYANOSTEROIDS AND THEIR BIOLOGICAL ACTIVITIES – A BRIEF REVIEW Article de journal
Dans: vol. 6, no. 12, p. 127–151, 2017, ISBN: 2017121061.
@article{Levitsky2017,
title = {ANABOLIC CYANOSTEROIDS AND THEIR BIOLOGICAL ACTIVITIES – A BRIEF REVIEW},
author = {Dmitri O Levitsky and Tatyana A Gloriozova and Vladimir V Poroikov and M Valery},
doi = {10.20959/wjpps201712-10618},
isbn = {2017121061},
year = {2017},
date = {2017-01-01},
volume = {6},
number = {12},
pages = {127--151},
abstract = {The present review describes the biological activities of synthetic anabolic cyanosteroids. More than forty biologically active compounds have shown confirmed anti-tumour, anti-inflammatory, antiviral and other activities. The structures and reported and predicted activities of synthetic cyanosteroids are available. With the computer programme PASS and based on structure–activity relationships (SAR), some additional activities are also predicted, which point towards new possible applications of these lipids. This review emphasizes the role of cyanosteroids as an important source and potential leads for drug discovery and they are of great interest to chemists, physicians, biologists, pharmacologists and the pharmaceutical industry. KEYWORDS:},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Faucon, Adrien; Benhelli-Mokrani, Houda; Fleury, Fabrice; Dutertre, Stéphanie; Tramier, Marc; Boucard, Joanna; Lartigue, Lénaïc; Nedellec, Steven; Hulin, Philippe; Ishow, Eléna
Bioconjugated fluorescent organic nanoparticles targeting EGFR-overexpressing cancer cells Article de journal
Dans: Nanoscale, vol. 9, no. 45, p. 18094–18106, 2017, ISSN: 20403372.
@article{Faucon2017,
title = {Bioconjugated fluorescent organic nanoparticles targeting EGFR-overexpressing cancer cells},
author = {Adrien Faucon and Houda Benhelli-Mokrani and Fabrice Fleury and Stéphanie Dutertre and Marc Tramier and Joanna Boucard and Lénaïc Lartigue and Steven Nedellec and Philippe Hulin and Eléna Ishow},
doi = {10.1039/c7nr06533g},
issn = {20403372},
year = {2017},
date = {2017-01-01},
journal = {Nanoscale},
volume = {9},
number = {45},
pages = {18094--18106},
publisher = {Royal Society of Chemistry},
abstract = {The field of optical bioimaging has considerably flourished with the advent of sophisticated microscopy techniques and ultra-bright fluorescent tools. Fluorescent organic nanoparticles (FONs) have thus recently appeared as very attractive labels for their high payload, absence of cytotoxicity and eventual biodegradation. Nevertheless, their bioconjugation to target specific receptors with high imaging contrast is scarcely performed. Moreover, assessing the reality of bioconjugation represents high challenges given the sub-nanomolar concentrations resulting from the commonly adopted nanoprecipitation fabrication process. Here, we describe how the combination of a magnetic shell allows us to easily generate red-emitting FONs conjugated with the epidermal growth factor ligand (EGF), a small protein promoting cancer cell proliferation by activating the EGF receptor (EGFR) pathway. Dual color fluorescence correlation spectroscopy combined with immunofluorescence is originally harnessed in its time trace mode to unambiguously demonstrate covalent attachment between the FON and EGF at sub-nanomolar concentrations. Strong asymmetric clustering of EGF-conjugated FONs is observed at the membrane of MDA-MB-468 human breast cancer cells overexpressing EGF receptors using super-resolution fluorescence microscopy. Such high recruitment of EGF-conjugated FONs is attributed to their EGF multivalency (4.7 EGF per FON) which enables efficient EGFR activation and subsequent phosphorylation. The large hydrodynamic diameter (DH ∼ 301 nm) of EGF-conjugated FONs prevents immediate engulfment of the sequestered receptors, which provides very bright and localized spots in less than 30 minutes. The reported bioconjugated nanoassemblies could thus serve as ultra-bright probes of breast cancer cells with EGFR-overexpression that is often associated with poor prognosis.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Alligand, Brendan; Breton, Magali Le; Marquis, Damien; Vallette, François; Fleury, Fabrice
Functional effects of diphosphomimetic mutations at cAbl-mediated phosphorylation sites on Rad51 recombinase activity Article de journal
Dans: Biochimie, vol. 139, p. 115–124, 2017, ISSN: 61831638.
@article{Alligand2017,
title = {Functional effects of diphosphomimetic mutations at cAbl-mediated phosphorylation sites on Rad51 recombinase activity},
author = {Brendan Alligand and Magali {Le Breton} and Damien Marquis and François Vallette and Fabrice Fleury},
url = {http://dx.doi.org/10.1016/j.biochi.2017.05.020},
doi = {10.1016/j.biochi.2017.05.020},
issn = {61831638},
year = {2017},
date = {2017-01-01},
journal = {Biochimie},
volume = {139},
pages = {115--124},
publisher = {Elsevier Ltd},
abstract = {Homologous Recombination enables faithful repair of the deleterious double strand breaks of DNA. This pathway relies on Rad51 to catalyze homologous DNA strand exchange. Rad51 is known to be phosphorylated in a sequential manner on Y315 and then on Y54, but the effect of such phosphorylation on Rad51 function remains poorly understood. We have developed a phosphomimetic model in order to study all the phosphorylation states. With the purified phosphomimetic proteins we performed in vitro assays to determine the activity of Rad51. Here we demonstrate the inhibitory effect of the double phosphomimetic mutant and suggest that it may be due to a defect in nucleofilament formation.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
9 publications
Silva, Viviane A O; Lafont, Florian; Benhelli-Mokrani, Houda; Breton, Magali Le; Hulin, Philippe; Chabot, Thomas; Paris, François; Sakanyan, Vehary; Fleury, Fabrice
Rapid diminution in the level and activity of DNA-dependent protein kinase in cancer cells by a reactive nitro-benzoxadiazole compound Article de journal
Dans: International Journal of Molecular Sciences, vol. 17, no. 5, 2016, ISSN: 14220067.
@article{Silva2016,
title = {Rapid diminution in the level and activity of DNA-dependent protein kinase in cancer cells by a reactive nitro-benzoxadiazole compound},
author = {Viviane A O Silva and Florian Lafont and Houda Benhelli-Mokrani and Magali {Le Breton} and Philippe Hulin and Thomas Chabot and Fran{ç}ois Paris and Vehary Sakanyan and Fabrice Fleury},
doi = {10.3390/ijms17050703},
issn = {14220067},
year = {2016},
date = {2016-05-01},
journal = {International Journal of Molecular Sciences},
volume = {17},
number = {5},
publisher = {MDPI AG},
abstract = {The expression and activity of DNA-dependent protein kinase (DNA-PK) is related to DNA repair status in the response of cells to exogenous and endogenous factors. Recent studies indicate that Epidermal Growth Factor Receptor (EGFR) is involved in modulating DNA-PK. It has been shown that a compound 4-nitro-7-[(1-oxidopyridin-2-yl)sulfanyl]-2,1,3-benzoxadiazole (NSC), bearing a nitro-benzoxadiazole (NBD) scaffold, enhances tyrosine phosphorylation of EGFR and triggers downstream signaling pathways. Here, we studied the behavior of DNA-PK and other DNA repair proteins in prostate cancer cells exposed to compound NSC. We showed that both the expression and activity of DNA-PKcs (catalytic subunit of DNA-PK) rapidly decreased upon exposure of cells to the compound. The decline in DNA-PKcs was associated with enhanced protein ubiquitination, indicating the activation of cellular proteasome. However, pretreatment of cells with thioglycerol abolished the action of compound NSC and restored the level of DNA-PKcs. Moreover, the decreased level of DNA-PKcs was associated with the production of intracellular hydrogen peroxide by stable dimeric forms of Cu/Zn SOD1 induced by NSC. Our findings indicate that reactive oxygen species and electrophilic intermediates, generated and accumulated during the redox transformation of NBD compounds, are primarily responsible for the rapid modulation of DNA-PKcs functions in cancer cells.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Krylov, Igor B; Kompanets, Mykhailo O; Novikova, Katerina V; Opeida, Iosip O; Kushch, Olga V; Shelimov, Boris N; Nikishin, Gennady I; Levitsky, Dmitri O; Terent'ev, Alexander O
Well-Known Mediators of Selective Oxidation with Unknown Electronic Structure: Metal-Free Generation and EPR Study of Imide-N-oxyl Radicals Article de journal
Dans: Journal of Physical Chemistry A, vol. 120, no. 1, p. 68–73, 2016, ISSN: 15205215.
@article{Krylov2016,
title = {Well-Known Mediators of Selective Oxidation with Unknown Electronic Structure: Metal-Free Generation and EPR Study of Imide-N-oxyl Radicals},
author = {Igor B Krylov and Mykhailo O Kompanets and Katerina V Novikova and Iosip O Opeida and Olga V Kushch and Boris N Shelimov and Gennady I Nikishin and Dmitri O Levitsky and Alexander O Terent'ev},
doi = {10.1021/acs.jpca.5b10722},
issn = {15205215},
year = {2016},
date = {2016-01-01},
journal = {Journal of Physical Chemistry A},
volume = {120},
number = {1},
pages = {68--73},
abstract = {Nitroxyl radicals are widely used in chemistry, materials sciences, and biology. Imide-N-oxyl radicals are subclass of unique nitroxyl radicals that proved to be useful catalysts and mediators of selective oxidation and CH-functionalization. An efficient metal-free method was developed for the generation of imide-N-oxyl radicals from N-hydroxyimides at room temperature by the reaction with (diacetoxyiodo)benzene. The method allows for the production of high concentrations of free radicals and provides high resolution of their EPR spectra exhibiting the superhyperfine structure from benzene ring protons distant from the radical center. An analysis of the spectra shows that, regardless of the electronic effects of the substituents in the benzene ring, the superhyperfine coupling constant of an unpaired electron with the distant protons at positions 4 and 5 of the aromatic system is substantially greater than that with the protons at positions 3 and 6 that are closer to the N-oxyl radical center. This is indicative of an unusual character of the spin density distribution of the unpaired electron in substituted phthalimide-N-oxyl radicals. Understanding of the nature of the electron density distribution in imide-N-oxyl radicals may be useful for the development of commercial mediators of oxidation based on N-hydroxyimides.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Mansuroglu, Zeyni; Benhelli-Mokrani, Houda; Marcato, Vasco; Sultan, Audrey; Violet, Marie; Chauderlier, Alban; Delattre, Lucie; Loyens, Anne; Talahari, Smail; Bégard, Séverine; Nesslany, Fabrice; Colin, Morvane; Souès, Sylvie; Lefebvre, Bruno; Buée, Luc; Galas, Marie Christine; Bonnefoy, Eliette
Loss of Tau protein affects the structure, transcription and repair of neuronal pericentromeric heterochromatin Article de journal
Dans: Scientific Reports, vol. 6, no. September, p. 1–16, 2016, ISSN: 20452322.
@article{Mansuroglu2016,
title = {Loss of Tau protein affects the structure, transcription and repair of neuronal pericentromeric heterochromatin},
author = {Zeyni Mansuroglu and Houda Benhelli-Mokrani and Vasco Marcato and Audrey Sultan and Marie Violet and Alban Chauderlier and Lucie Delattre and Anne Loyens and Smail Talahari and Séverine Bégard and Fabrice Nesslany and Morvane Colin and Sylvie Souès and Bruno Lefebvre and Luc Buée and Marie Christine Galas and Eliette Bonnefoy},
doi = {10.1038/srep33047},
issn = {20452322},
year = {2016},
date = {2016-01-01},
journal = {Scientific Reports},
volume = {6},
number = {September},
pages = {1--16},
publisher = {Nature Publishing Group},
abstract = {Pericentromeric heterochromatin (PCH) gives rise to highly dense chromatin sub-structures rich in the epigenetic mark corresponding to the trimethylated form of lysine 9 of histone H3 (H3K9me3) and in heterochromatin protein 1α (HP1α), which regulate genome expression and stability. We demonstrate that Tau, a protein involved in a number of neurodegenerative diseases including Alzheimer's disease (AD), binds to and localizes within or next to neuronal PCH in primary neuronal cultures from wild-type mice. Concomitantly, we show that the clustered distribution of H3K9me3 and HP1α, two hallmarks of PCH, is disrupted in neurons from Tau-deficient mice (KOTau). Such altered distribution of H3K9me3 that could be rescued by overexpressing nuclear Tau protein was also observed in neurons from AD brains. Moreover, the expression of PCH non-coding RNAs, involved in PCH organization, was disrupted in KOTau neurons that displayed an abnormal accumulation of stress-induced PCH DNA breaks. Altogether, our results demonstrate a new physiological function of Tau in directly regulating neuronal PCH integrity that appears disrupted in AD neurons.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Jaunet-Lahary, Titouan; Goupille, Anaïs; Jacquemin, Denis; Fleury, Fabrice; Graton, Jérôme; Laurent, Adèle D
A Joint Theoretical and Experimental Study of the Behavior of the DIDS Inhibitor and its Derivatives Article de journal
Dans: ChemPhysChem, vol. 3, p. 2434–2445, 2016, ISSN: 14397641.
@article{Jaunet-Lahary2016,
title = {A Joint Theoretical and Experimental Study of the Behavior of the DIDS Inhibitor and its Derivatives},
author = {Titouan Jaunet-Lahary and Anaïs Goupille and Denis Jacquemin and Fabrice Fleury and Jérôme Graton and Adèle D Laurent},
doi = {10.1002/cphc.201600107},
issn = {14397641},
year = {2016},
date = {2016-01-01},
journal = {ChemPhysChem},
volume = {3},
pages = {2434--2445},
abstract = {4,4′-Diisothiocyanostilbene-2,2′-disulfonic acid (DIDS) is a well-known ion-exchange inhibitor targeting cardiac functions and indirectly impeding both radio- and chemo-resistance. A joint computational and experimental study is presented to provide deeper insights into DIDS and other members of this family of compounds. To this end, we applied state-of-the-art density functional theory (DFT) and time-dependent DFT methods, in addition to measuring the optical properties. The experimental data show that such compounds are highly sensitive to their environment and that the optical properties change within as little time as 7 h. However, the optical properties of DIDS are similar in various acidic/basic environments, which were confirmed by pKa computations on both cis and trans isomers. The protonation analysis also highlights that the singly protonated form of DIDS behaves like a proton sponge compound. The experimentally observed redshift that can be seen when going from water to DMSO was reproduced solely by using the solvation model based on density, although the polarization continuum model and implicit/explicit hybrid schemes were also tested. The characteristic broadening of the absorption peak in water and the vibronic fine structure in DMSO were also reproduced thanks to vibronic coupling simulations associated with the solvent reorganization energy. For other stilbene derivatives, a correlation is found between the maximum absorption wavelength and the Hammett parameters.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Terent'Ev, Alexander O; Pastukhova, Zhanna Yu; Yaremenko, Ivan A; Novikov, Roman A; Demchuk, Dmitry V; Bruk, Lev G; Levitsky, Dmitri O; Fleury, Fabrice; Nikishin, Gennady I
Selective transformation of tricyclic peroxides with pronounced antischistosomal activity into 2-hydroxy-1,5-diketones using iron (II) salts Article de journal
Dans: Tetrahedron, vol. 72, no. 24, p. 3421–3426, 2016, ISSN: 14645416.
@article{TerentEv2016,
title = {Selective transformation of tricyclic peroxides with pronounced antischistosomal activity into 2-hydroxy-1,5-diketones using iron (II) salts},
author = {Alexander O Terent'Ev and Zhanna Yu Pastukhova and Ivan A Yaremenko and Roman A Novikov and Dmitry V Demchuk and Lev G Bruk and Dmitri O Levitsky and Fabrice Fleury and Gennady I Nikishin},
url = {http://dx.doi.org/10.1016/j.tet.2016.04.054},
doi = {10.1016/j.tet.2016.04.054},
issn = {14645416},
year = {2016},
date = {2016-01-01},
journal = {Tetrahedron},
volume = {72},
number = {24},
pages = {3421--3426},
publisher = {Elsevier Ltd},
abstract = {The present work deals with selective transformations of peroxides into organic compounds via the cleavage of the O-O bond using variable valence metals. A selective transformation of tricyclic peroxides promoted by Fe2+ salts was discovered. This selective transformation is unexpected for compounds with structural features which allow diverse decomposition pathways. 2-Hydroxy-1,5-diketones are prepared in yields up to 92% in the reactions of tricyclic peroxides with FeSO4, Fe(ClO4)2, or FeCl2. This is a new preparative method for the synthesis of 1,5-diketones. 2-Hydroxy-1,5-diketones in CDCl3 at 25 °C exist mainly in the open-chain form of the hydroxyketone over the cyclic hemiacetal. The results of this work can be of interest to understand the mechanism of the antiparasitic action of peroxides.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Levitsky, Dmitri O; Gloriozova, Tatyana A; Poroikov, Vladimir V; Dembitsky, Valery M
Mathews Journal of Pharmaceutical Science Naturally Occurring Isocyano / Isothiocyanato Compounds : Their Pharmacological and SAR Activities Article de journal
Dans: MATHEWS, Open Access Journals, vol. 1, no. 1, p. 1–15, 2016.
@article{Levitsky2016,
title = {Mathews Journal of Pharmaceutical Science Naturally Occurring Isocyano / Isothiocyanato Compounds : Their Pharmacological and SAR Activities},
author = {Dmitri O Levitsky and Tatyana A Gloriozova and Vladimir V Poroikov and Valery M Dembitsky},
year = {2016},
date = {2016-01-01},
journal = {MATHEWS, Open Access Journals},
volume = {1},
number = {1},
pages = {1--15},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Zdvizhkov, Alexander T; Terent'Ev, Alexander O; Radulov, Peter S; Novikov, Roman A; Tafeenko, Viktor A; Chernyshev, Vladimir V; Ilovaisky, Alexey I; Levitsky, Dmitri O; Fleury, Fabrice; Nikishin, Gennady I
Transformation of 2-allyl-1,3-diketones to bicyclic compounds containing 1,2-dioxolane and tetrahydrofuran rings using the I2/H2O2 system Article de journal
Dans: Tetrahedron Letters, vol. 57, no. 8, p. 949–952, 2016, ISSN: 18733581.
@article{Zdvizhkov2016,
title = {Transformation of 2-allyl-1,3-diketones to bicyclic compounds containing 1,2-dioxolane and tetrahydrofuran rings using the I2/H2O2 system},
author = {Alexander T Zdvizhkov and Alexander O Terent'Ev and Peter S Radulov and Roman A Novikov and Viktor A Tafeenko and Vladimir V Chernyshev and Alexey I Ilovaisky and Dmitri O Levitsky and Fabrice Fleury and Gennady I Nikishin},
url = {http://dx.doi.org/10.1016/j.tetlet.2016.01.061},
doi = {10.1016/j.tetlet.2016.01.061},
issn = {18733581},
year = {2016},
date = {2016-01-01},
journal = {Tetrahedron Letters},
volume = {57},
number = {8},
pages = {949--952},
publisher = {Elsevier Ltd},
abstract = {A one-pot procedure was developed for the assembly of bicyclic compounds containing 1,2-dioxolane and tetrahydrofuran rings based on the reaction of 2-allyl-1,3-diketones with the I2/H2O2 system. A fivefold molar excess of H2O2 and a twofold excess of I2 are required for the selective formation of tetrahydrofurodioxoles. The synthesis of these structurally complex molecules is unusual in that it does not produce the expected bridged tetraoxanes, products of the addition of several H2O2 molecules to a carbonyl group, or the products of double bond iodoperoxidation.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Sakanyan, Vehary; Hulin, Philippe; Sousa, Rodolphe Alves De; Silva, Viviane A O; Hambardzumyan, Artur; Nedellec, Steven; Tomasoni, Christophe; Logé, Cédric; Pineau, Charles; Roussakis, Christos; Fleury, Fabrice; Artaud, Isabelle
Activation of EGFR by small compounds through coupling the generation of hydrogen peroxide to stable dimerization of Cu/Zn SOD1 Article de journal
Dans: Scientific Reports, vol. 6, no. January, p. 1–14, 2016, ISSN: 20452322.
@article{Sakanyan2016,
title = {Activation of EGFR by small compounds through coupling the generation of hydrogen peroxide to stable dimerization of Cu/Zn SOD1},
author = {Vehary Sakanyan and Philippe Hulin and Rodolphe {Alves De Sousa} and Viviane A O Silva and Artur Hambardzumyan and Steven Nedellec and Christophe Tomasoni and Cédric Logé and Charles Pineau and Christos Roussakis and Fabrice Fleury and Isabelle Artaud},
url = {http://dx.doi.org/10.1038/srep21088},
doi = {10.1038/srep21088},
issn = {20452322},
year = {2016},
date = {2016-01-01},
journal = {Scientific Reports},
volume = {6},
number = {January},
pages = {1--14},
publisher = {Nature Publishing Group},
abstract = {Activation of cell signaling by reactive chemicals and pollutants is an important issue for human health. It has been shown that lipophilic nitro-benzoxadiazole (NBD) compounds rapidly move across the plasma membrane and enhance Epidermal Growth Factor Receptor (EGFR) tyrosine phosphorylation in cancer cells. Unlike ligand-dependent activation, the mechanism of this induction relies on the generation of hydrogen peroxide, which is involved in the activation of the catalytic site of the receptor and the inactivation of protein tyrosine phosphatase PTP-1B. Production of H 2 O 2 during redox transformation of NBD compounds is associated with the transition of a monomeric form of Cu/Zn superoxide dismutase 1 (SOD1) to stable dimers. The highly stable and functionally active SOD1 dimer, in the absence of adequate activities in downstream reactions, promotes the disproportionate production and accumulation of intracellular hydrogen peroxide shortly after exposure to NBD compounds. The intrinsic fluorescence of small compounds was used to demonstrate their binding to SOD1. Our data indicate that H 2 O 2 and concomitantly generated electrophilic intermediates behave as independent entities, but all contribute to the biological reactivity of NBD compounds. This study opens a promising path to identify new biomarkers of oxidative/electrophilic stress in the progression of cancer and other diseases.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Faucon, Adrien; Benhelli-Mokrani, Houda; Fleury, Fabrice; Dubreil, Laurence; Hulin, Philippe; Nedellec, Steven; Doussineau, Tristan; Antoine, Rodolphe; Orlando, Tomas; Lascialfari, Alessandro; Fresnais, Jérôme; Lartigue, Lénaïc; Ishow, Eléna
Tuning the architectural integrity of high-performance magneto-fluorescent core-shell nanoassemblies in cancer cells Article de journal
Dans: Journal of Colloid and Interface Science, vol. 479, p. 139–149, 2016, ISSN: 10957103.
@article{Faucon2016,
title = {Tuning the architectural integrity of high-performance magneto-fluorescent core-shell nanoassemblies in cancer cells},
author = {Adrien Faucon and Houda Benhelli-Mokrani and Fabrice Fleury and Laurence Dubreil and Philippe Hulin and Steven Nedellec and Tristan Doussineau and Rodolphe Antoine and Tomas Orlando and Alessandro Lascialfari and Jér{ô}me Fresnais and Léna{ï}c Lartigue and Eléna Ishow},
url = {http://dx.doi.org/10.1016/j.jcis.2016.06.064},
doi = {10.1016/j.jcis.2016.06.064},
issn = {10957103},
year = {2016},
date = {2016-01-01},
journal = {Journal of Colloid and Interface Science},
volume = {479},
pages = {139--149},
publisher = {Elsevier Inc.},
abstract = {High-density nanoarchitectures, endowed with simultaneous fluorescence and contrast properties for MRI and TEM imaging, have been obtained using a simple self-assembling strategy based on supramolecular interactions between non-doped fluorescent organic nanoparticles (FON) and superparamagnetic nanoparticles. In this way, a high-payload core-shell structure FON@mag has been obtained, protecting the hydrophobic fluorophores from the surroundings as well as from emission quenching by the shell of magnetic nanoparticles. Compared to isolated nanoparticles, maghemite nanoparticles self-assembled as an external shell create large inhomogeneous magnetic field, which causes enhanced transverse relaxivity and exacerbated MRI contrast. The magnetic load of the resulting nanoassemblies is evaluated using magnetic sedimentation and more originally electrospray mass spectrometry. The role of the stabilizing agents (citrate versus polyacrylate anions) revealed to be crucial regarding the cohesion of the resulting high-performance magneto-fluorescent nanoassemblies, which questions their use after cell internalization as nanocarriers or imaging agents for reliable correlative light and electron microcopy.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
5 publications
Alligand, Brendan
Étude du rôle des phosphorylations de Rad51 en Y54 et en Y315 sur son fonctionnement Thèse
Université de Nantes, 2015.
@phdthesis{alligand2015etude,
title = {Étude du rôle des phosphorylations de Rad51 en Y54 et en Y315 sur son fonctionnement},
author = {Brendan Alligand},
url = {https://www.theses.fr/2015NANT2033},
year = {2015},
date = {2015-11-26},
school = {Université de Nantes},
abstract = {La Recombinaison Homologue (RH) permet la réparation des dommages à l’ADN les plus délétères : les Cassures double brin. L’étape centrale de la RH est basée sur l’activité d’échange de brins de RAD51. Ainsi, l’activité de RAD51 est cruciale pour le maintien de l’intégrité génomique. Toutefois, cette protéine possède également un côté sombre. En effet, la surexpression de RAD51 permet aux cellules cancéreuses de résister aux traitements. Ce qui en fait une cible thérapeutique potentielle pour sensibiliser les cellules cancéreuses au traitement. Une meilleure compréhension du contrôle de l’activité de RAD51 aiderait sûrement à développer des stratégies thérapeutiques. L’activité de RAD51 est régulée par des phosphorylations et plusieurs kinases sont connues pour cibler RAD51. C’est le cas de la kinase c-Abl qui phosphoryle les tyrosines Y54 et Y315 en réponse aux dommages à l’ADN. Mais le rôle de ces phosphorylations est peu connu. C’est pourquoi nous nous sommes intéressés à l’effet de ces phosphorylations sur RAD51. Dans ce but, nous avons produit des mutants de RAD51 mimant la phosphorylation. Leur activité a été analysée et comparée in vitro. Nous avons démontré que le mutant équivalent à une double phosphorylation est incapable de réaliser l’échange de brins. Un défaut de polymérisation de RAD51 serait à l’origine de cette inhibition. Par la suite, la régulation a été étudiée dans le contexte cellulaire. Les résultats préliminaires montrent un effet de la double phosphorylation sur la localisation cellulaire de RAD51. L’inactivation de RAD51 par cette double phosphorylation pourrait participer à la régulation de la voie de la Recombinaison Homologue et serait une étape clef dans la compréhension de la réponse aux dommages à l’ADN.},
keywords = {},
pubstate = {published},
tppubtype = {phdthesis}
}
Faucon, Adrien; Benhelli-Mokrani, Houda; w Córdova, Luis A; Brulin, Bénédicte; Heymann, Dominique; Hulin, Philippe; Nedellec, Steven; Ishow, Eléna
Are Fluorescent Organic Nanoparticles Relevant Tools for Tracking Cancer Cells or Macrophages? Article de journal
Dans: Advanced Healthcare Materials, vol. 4, no. 17, p. 2727–2734, 2015, ISSN: 21922659.
@article{Faucon2015,
title = {Are Fluorescent Organic Nanoparticles Relevant Tools for Tracking Cancer Cells or Macrophages?},
author = {Adrien Faucon and Houda Benhelli-Mokrani and Luis A w Córdova and Bénédicte Brulin and Dominique Heymann and Philippe Hulin and Steven Nedellec and Eléna Ishow},
doi = {10.1002/adhm.201500562},
issn = {21922659},
year = {2015},
date = {2015-01-01},
journal = {Advanced Healthcare Materials},
volume = {4},
number = {17},
pages = {2727--2734},
abstract = {Strongly solvatochromic fluorophores are devised, containing alkyl chains and enable to self-assemble as very bright fluorescent organic nanoparticles (FONs) in water (φf = 0.28). The alkyl chains impart each fluorophore with strongly hydrophobic surroundings, causing distinct emission colors between FONs where the fluorophores are associated, and their disassembled state. Such color change is harnessed to assess the long-term fate of FONs in both cancer cells and monocytes/macrophages. Disintegration of the orange-emitting FONs by monocytes/macrophages is evidenced through the formation of micrometer green-yellowish emitting vesicles. By contrast, cancer cells retain longer the integrity of organic nanoparticles. In both cases, no significant toxicity is detected, making FONs as valuable bioimaging agents for cell tracking with weak risks of deleterious accumulation and low degradation rate. Long-term fate of fluorescent organic nanoparticles (FONs), known as very bright imaging agents and made of self-assembled solvatochromic fluorophores, is explored in both cancer cells and monocytes/macrophages. Disintegration of the orange-emitting FONs by monocytes/macrophages is evidenced through the formation of micrometer green-yellowish emitting vesicles. By contrast, cancer cells retain longer the integrity of organic nanoparticles.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Qi, Haoling; Cantrelle, François Xavier; Benhelli-Mokrani, Houda; Smet-Nocca, Caroline; Buée, Luc; Lippens, Guy; Bonnefoy, Eliette; Galas, Marie Christine; Landrieu, Isabelle
Nuclear magnetic resonance spectroscopy characterization of interaction of Tau with DNA and its regulation by phosphorylation Article de journal
Dans: Biochemistry, vol. 54, no. 7, p. 1525–1533, 2015, ISSN: 15204995.
@article{Qi2015,
title = {Nuclear magnetic resonance spectroscopy characterization of interaction of Tau with DNA and its regulation by phosphorylation},
author = {Haoling Qi and François Xavier Cantrelle and Houda Benhelli-Mokrani and Caroline Smet-Nocca and Luc Buée and Guy Lippens and Eliette Bonnefoy and Marie Christine Galas and Isabelle Landrieu},
doi = {10.1021/bi5014613},
issn = {15204995},
year = {2015},
date = {2015-01-01},
journal = {Biochemistry},
volume = {54},
number = {7},
pages = {1525--1533},
abstract = {The capacity of endogenous Tau to bind DNA has been recently identified in neurons under physiological or oxidative stress conditions. Characterization of the protein domains involved in Tau-DNA complex formation is an essential first step in clarifying the contribution of Tau-DNA interactions to neurological biological processes. To identify the amino acid residues involved in the interaction of Tau with oligonucleotides, we have characterized a Tau-DNA complex using nuclear magnetic resonance spectroscopy. Interaction of an AT-rich or GC-rich 22 bp oligonucleotide with Tau showed multiple points of anchoring along the intrinsically disordered Tau protein. The main sites of contact characterized here correspond to the second half of the proline-rich domain (PRD) of Tau and the R2 repeat in the microtubule binding domain. This latter interaction site includes the PHF6∗ sequence known to govern Tau aggregation. The characterization was pursued by studying the binding of phosphorylated forms of Tau, displaying multiple phosphorylation sites mainly in the PRD, to the same oligonucleotide. No interaction of phospho-Tau with the oligonucleotide was detected, suggesting that pathological Tau phosphorylation could affect the physiological function of Tau mediated by DNA binding.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Velic, Denis; Couturier, Anthony M; Ferreira, Maria Tedim; Rodrigue, Amélie; Poirier, Guy G; Fleury, Fabrice; Masson, Jean-Yves
DNA Damage Signalling and Repair Inhibitors: The Long-Sought-After Achilles’ Heel of Cancer Article de journal
Dans: Biomolecules, vol. 5, no. 4, p. 3204–3259, 2015, ISSN: 2218-273X.
@article{biom5043204,
title = {DNA Damage Signalling and Repair Inhibitors: The Long-Sought-After Achilles’ Heel of Cancer},
author = {Denis Velic and Anthony M Couturier and Maria Tedim Ferreira and Amélie Rodrigue and Guy G Poirier and Fabrice Fleury and Jean-Yves Masson},
url = {https://www.mdpi.com/2218-273X/5/4/3204},
doi = {10.3390/biom5043204},
issn = {2218-273X},
year = {2015},
date = {2015-01-01},
journal = {Biomolecules},
volume = {5},
number = {4},
pages = {3204--3259},
abstract = {For decades, radiotherapy and chemotherapy were the two only approaches exploiting DNA repair processes to fight against cancer. Nowadays, cancer therapeutics can be a major challenge when it comes to seeking personalized targeted medicine that is both effective and selective to the malignancy. Over the last decade, the discovery of new targeted therapies against DNA damage signalling and repair has offered the possibility of therapeutic improvements in oncology. In this review, we summarize the current knowledge of DNA damage signalling and repair inhibitors, their molecular and cellular effects, and future therapeutic use.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Terent'ev, Alexander O; Zdvizhkov, Alexander T; Levitsky, Dmitri O; Fleury, Fabrice; Pototskiy, Roman A; Kulakova, Alena N; Nikishin, Gennady I
Organocatalytic peroxidation of malonates, β-ketoesters, and cyanoacetic esters using n-Bu4NI/t-BuOOH-mediated intermolecular oxidative C(sp3)-O coupling Article de journal
Dans: Tetrahedron, vol. 71, no. 47, p. 8985–8990, 2015, ISSN: 14645416.
@article{Terentev2015,
title = {Organocatalytic peroxidation of malonates, β-ketoesters, and cyanoacetic esters using n-Bu4NI/t-BuOOH-mediated intermolecular oxidative C(sp3)-O coupling},
author = {Alexander O Terent'ev and Alexander T Zdvizhkov and Dmitri O Levitsky and Fabrice Fleury and Roman A Pototskiy and Alena N Kulakova and Gennady I Nikishin},
url = {http://dx.doi.org/10.1016/j.tet.2015.09.047},
doi = {10.1016/j.tet.2015.09.047},
issn = {14645416},
year = {2015},
date = {2015-01-01},
journal = {Tetrahedron},
volume = {71},
number = {47},
pages = {8985--8990},
publisher = {Elsevier Ltd},
abstract = {A new organocatalytic approach for the synthesis of peroxides based on CH activation of a sp3-hybridized carbon atom is reported. Peroxides were prepared in 31-89% yield by the reaction of malonates, β-ketoesters, and cyanoacetic esters with a Bu4NI/tert-butyl hydroperoxide system. The formation of the expected hydroxylation products was not observed. In the discovered reaction, tert-butyl hydroperoxide plays a dual role by acting as the oxidant and the O-reagent for the C-O coupling. The synthesis can be scaled up to generate gram quantities of the target products.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}